Method for preparing iron phosphate from ferrophosphorus
A technology of ferric phosphate and ferrophosphorus, applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve the problems of no reports and patents of preparation methods, no discovery of ferric phosphate, etc., and meet low equipment requirements and production The effect of short process and easy operation and control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Prepare ferric phosphate from ferrophosphorus FeP by solid-phase method. Firstly, ferrophosphorus powder is crushed to a certain particle size, then 5g of ferrophosphorus powder is dried at 200°C, and then roasted in a dry air atmosphere at 500°C for 10 hours. Generated P 2 o 5 gas into Fe 2 o 3 In, after reacting for 10 hours, iron phosphate finished product is obtained, and the reaction equation is as follows:
[0023] 2FeP+4O 2 → Fe 2 o 3 +P 2 o 5
[0024] Fe 2 o 3 +P 2 o 5 →2FePO 4
[0025] In this reaction, the molar ratio of phosphorus and iron in the raw material ferrophosphorus is 1:1, and there is no need to supplement phosphorus or iron sources. It is only necessary to fully react in dry air after drying the ferrophosphorus powder. Adding other reaction raw materials, there is no serious high-temperature corrosion problem caused by phosphoric acid, the intermediate product of the first oxidation reaction can be completely reacted to form iron ph...
Embodiment 2
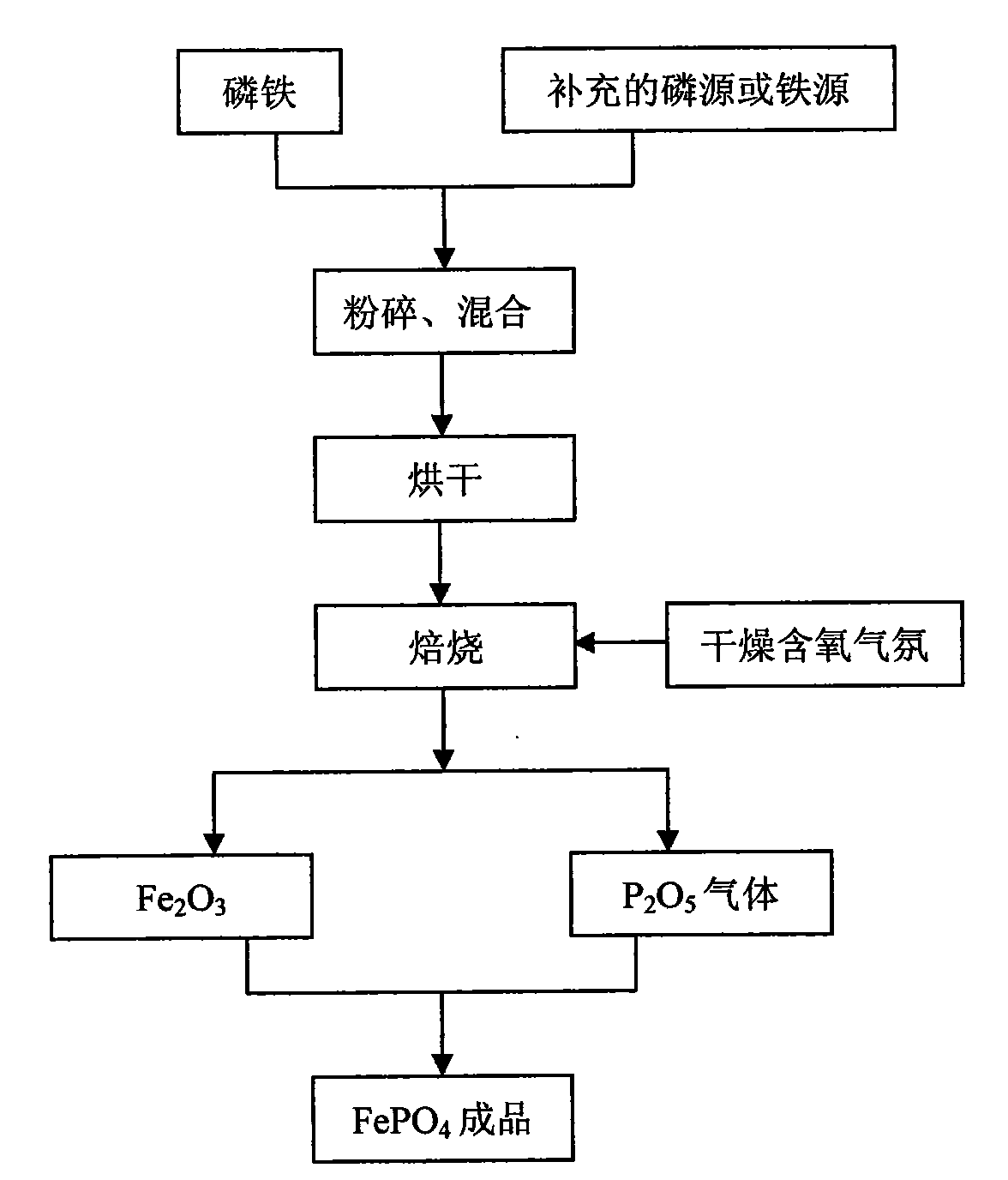
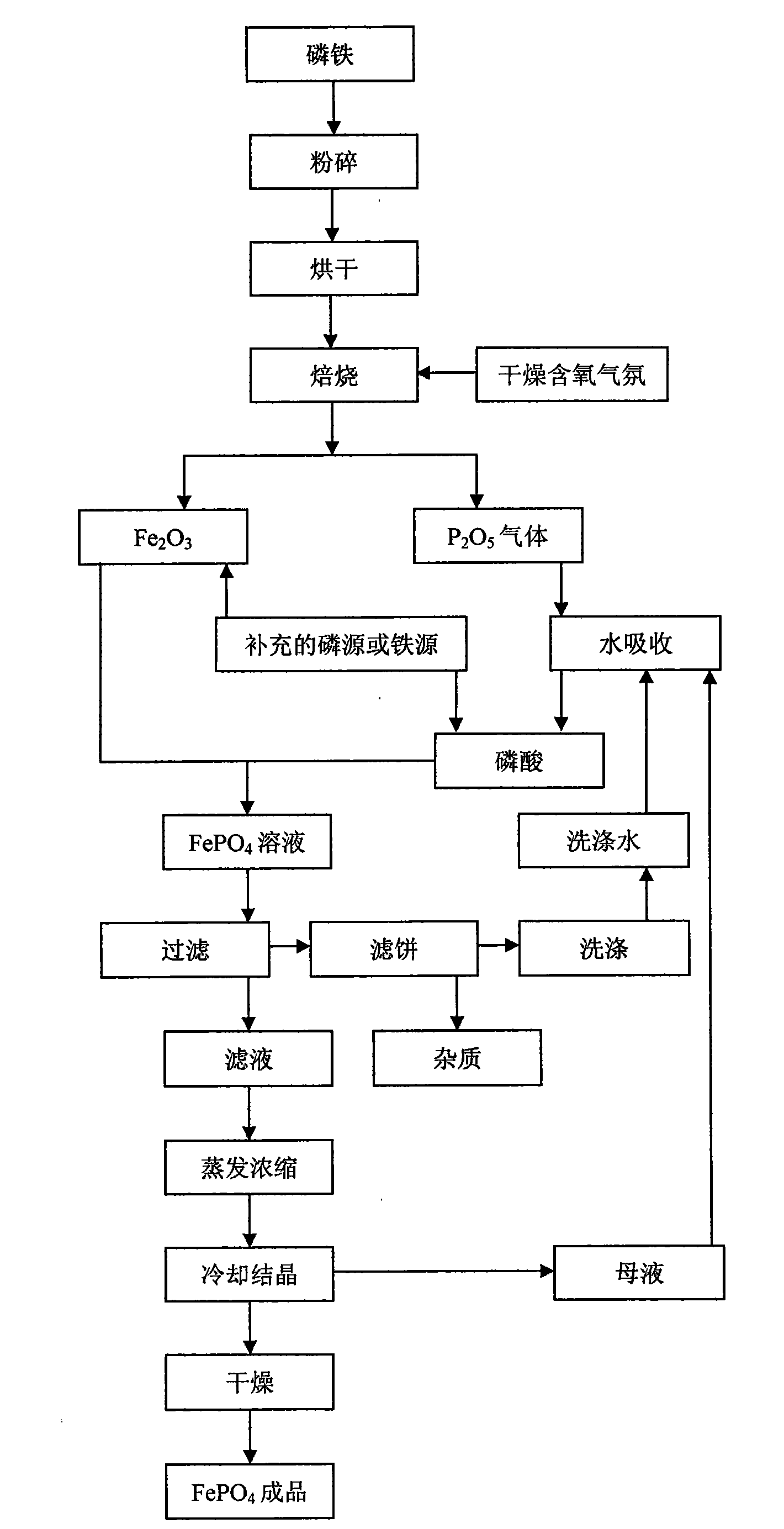
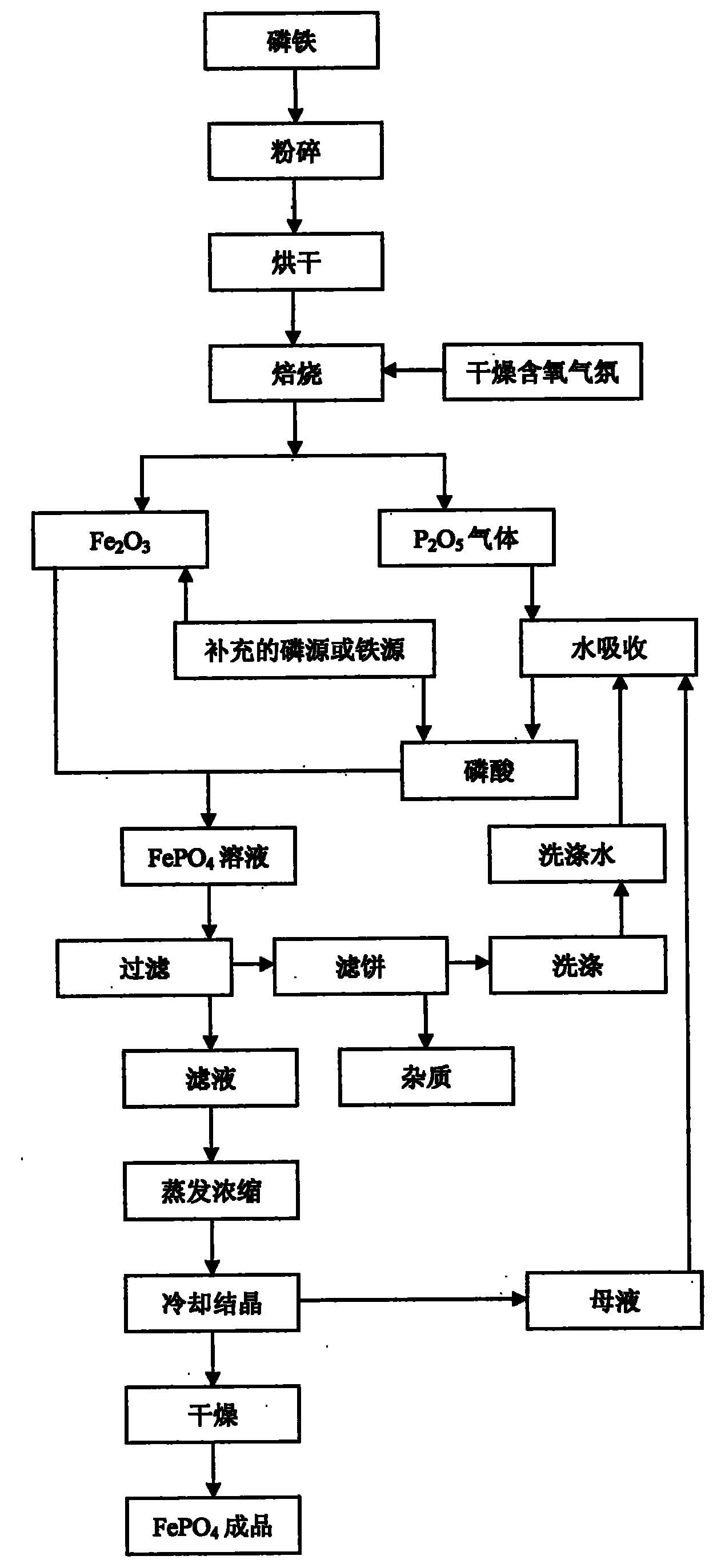
[0027] Ferrophosphorus Fe was prepared by atomization drying method 1.5 P prepares iron phosphate, selects P 2 o 5 In order to supplement the phosphorus source, add it before roasting, the molar ratio of the two is 4:1, first crush the ferrophosphorus to a certain particle size, then mix the ferrophosphorus powder with the added P 2 o 5 After the powder is atomized and dried, it is fired in a dry air atmosphere at 600°C for 7 hours, and the generated P 2 o 5 gas into Fe 2 o 3 In, after 12 hours of reaction, ferric phosphate finished product is obtained, and the technological process is as follows figure 1 As shown, the reaction equation is as follows:
[0028] 8Fe 1.5 P+19O 2 +2P 2 o 5 →6Fe 2 o 3 +6P 2 o 5
[0029] 6Fe 2 o 3 +6P 2 o 5 →12FePO 4
[0030] The reaction requires only the addition of P 2 o 5 As a supplementary phosphorus source, the molar ratio of total phosphorus and total iron in the reaction raw materials was adjusted to 1:1, and no other...
Embodiment 3
[0032] Ferrophosphorus FeP 3 Prepare ferric phosphate as raw material, use ferrophosphorus powder as phosphorus source and iron source, use Fe 3 o 4 In order to supplement the iron source, it is added before roasting, the molar ratio of the two is 3:2, and the ferrophosphorus powder and the supplementary iron source Fe 3 o 4 After mixing, it is ball milled at a speed of 300-600rpm in an oxygenated dry ball mill tank for 10 hours, and then roasted in the air at 700°C for 5 hours, using supplementary iron source, oxygen and oxygen in the air as iron phosphate. source of oxygen to produce FePO 4 Finished product, process such as figure 1 As shown, the reaction equation is as follows:
[0033] 3FeP 3 +2Fe 3 o 4 +14O 2 →9FePO 4
[0034] No other by-products are generated during the reaction, and the morphology and particle size distribution of the product can be controlled as required. The process is relatively simple, no other impurities are introduced, and it also has ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com