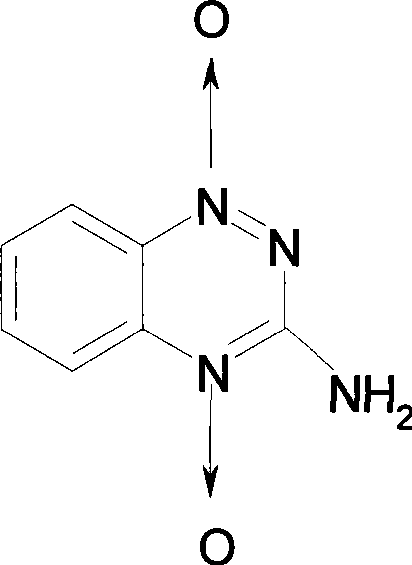
Tirapazamine parenteral hydrous preparation and preparation method thereof
A technology for tirapazamine and water preparations, which is applied in the directions of pharmaceutical formulas, drug combinations, active ingredients of heterocyclic compounds, etc., and can solve problems such as easy precipitation of tirapazamine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] A: Tirapazamine 0.712g
[0058] B: Sodium acetate 41g
[0059] C: 160ml glacial acetic acid
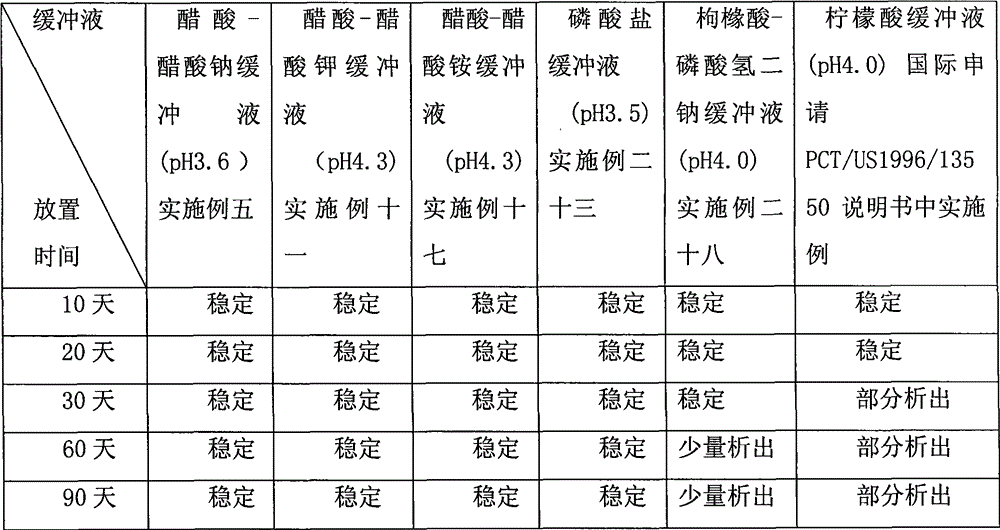
[0060] Dissolve B and C in about 500ml of water, raise the temperature appropriately to keep the solution temperature at about 60°C, slowly add A and stir to dissolve completely, cool to room temperature, add appropriate amount of water to 1000ml, the pH is about 2.5 . Fill and seal in 20ml ampoules, seal and pack.
[0061] Preparation purposes: intravenous injection.
Embodiment 2
[0063] A: Tirapazamine 0.0089g
[0064] B: Sodium acetate 0.4095g
[0065] C: Glacial acetic acid 1.605ml
[0066] Take about 500ml of water to dissolve B and C, raise the temperature appropriately to keep the solution temperature at about 50°C, slowly add A and stir to dissolve completely, after cooling to room temperature, add sodium chloride and other osmotic pressure regulators, add appropriate amount of water to 1000ml , pH is about 6.0.
[0067] Preparation purposes: intravenous infusion.
Embodiment 3
[0069] A: Tirapazamine 0.59g
[0070] B: Sodium acetate 27.3 grams
[0071] C: Glacial acetic acid 107ml
[0072] Dissolve B and C in about 500 ml of water, raise the temperature appropriately to keep the solution temperature at about 55°C, slowly add A and stir to dissolve completely, cool to room temperature, add appropriate amount of water to 1000 ml, and the pH is about 6.0. Fill and seal in 20ml ampoules, seal and pack.
[0073] Preparation use: intravenous injection
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com