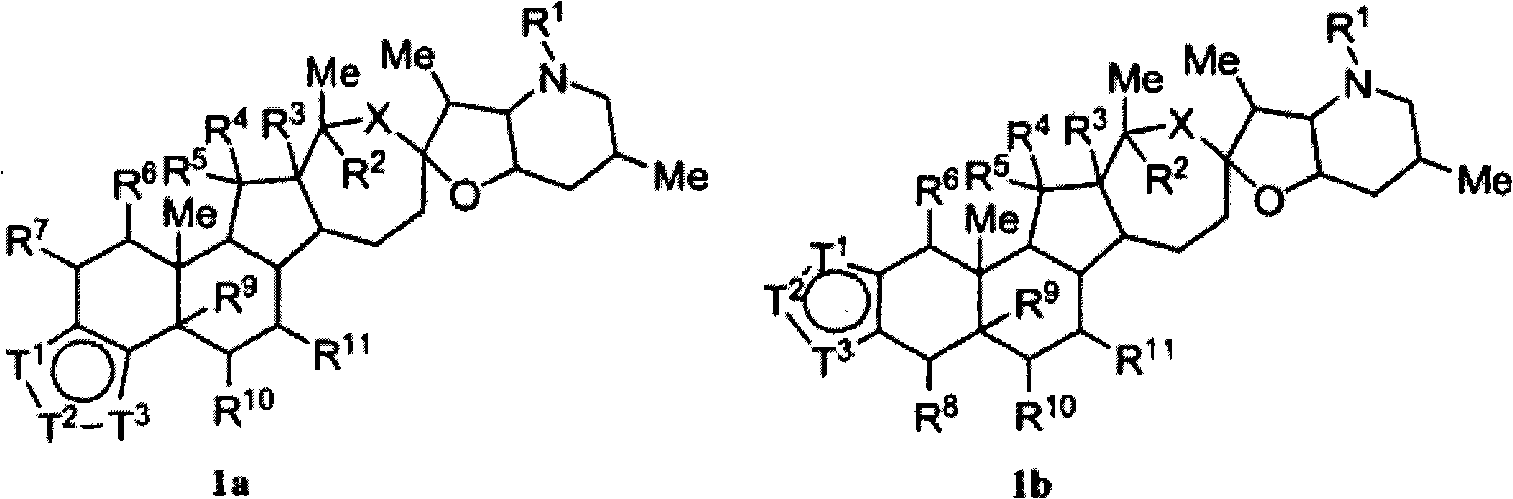
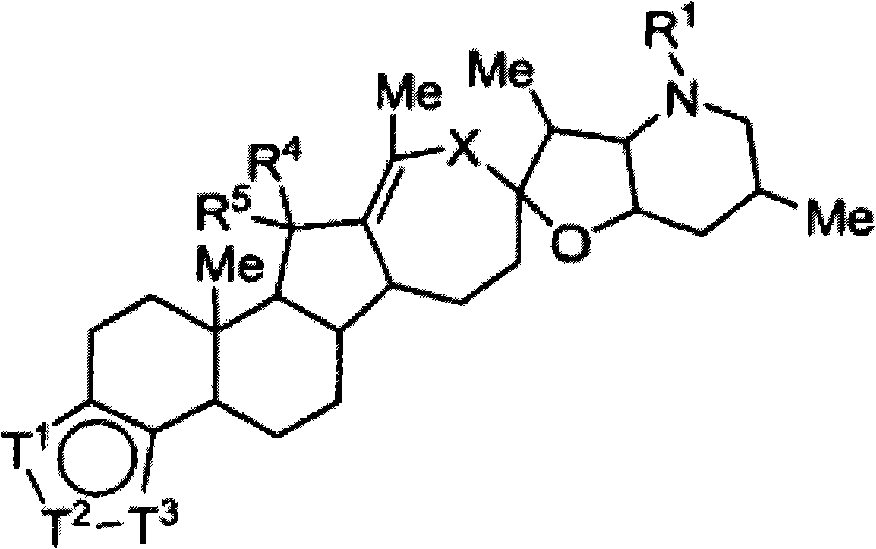
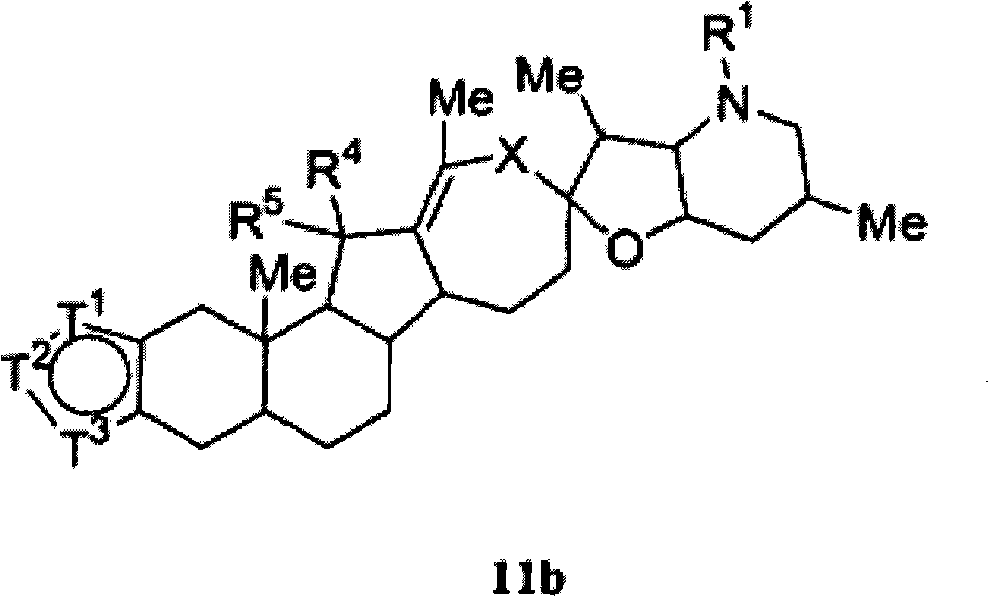
Heterocyclic cyclopamine analogs and methods of use thereof
A kind of compound, cycloalkyl technology, applied in the field of cyclopamine analogs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0194]
[0195] Step A
[0196]
[0197] Cyclopamine 2 (5.02 g, 12.2 mmol, 1.0 equiv) was dissolved in anhydrous pyridine (25 mL). DMAP (300 mg, 2.44 mmol, 0.2 equiv) and triethylamine (5.5 mL, 39.1 mmol, 3.2 equiv) were added, followed by BtO-Cbz (10.5 g, 39.1 mmol, 3.2 equiv) and the mixture was heated at 40 °C for 2 h . The mixture was cooled to room temperature, treated with 30 mL of water, heated to give a homogeneous solution, and cooled to room temperature. The white precipitate formed was collected by filtration, the filter cake was washed with water (3×50 mL) and air dried to give 9.53 g of crude product which was crystallized from toluene / heptane (1:9, 70 mL) to give 6.75 g of the desired product.
[0198] Step B:
[0199]
[0200]To a solution of diethylzinc (572 mg, 482 μL, 4.63 mmol, 3 equiv) in DCM (5.0 mL) was added bis(2,6-dimethylphenyl)phosphoric acid (1.42 g, 4.63 mmol) at -20 °C , 3 eq) in DCM (15 mL) while keeping the reaction temperature belo...
Embodiment 2
[0221]
[0222] A solution of compound 9 (100.0 mg, 0.17 mmol, 1.0 equiv) in ethanol (4 mL) was treated with hydrazine (16 mg, 0.34 mmol, 2.0 equiv) and heated at 70° C. for 0.5 hour. The mixture was concentrated in vacuo and purified by flash silica gel chromatography (20→60% ether / hexanes) to give the protected pyrazole (72.0 mg) as a white solid.
[0223] In a flask equipped with a stir bar and rubber septum, the product carbamate isoxazole was dissolved in EtOAc (7ml). The solution was bubbled with nitrogen and 10% Pd / C (wet, Degussa type E101, Aldrich, 25 mg) was added. The mixture was bubbled with nitrogen, then hydrogen and stirred at room temperature for 2 hours. The mixture was then bubbled with nitrogen, filtered through a 0.45 μm polyethylene membrane, and concentrated to a clear oil. The oil was purified by flash chromatography on silica gel (0.5% ammonium hydroxide / 2→10% MeOH / DCM), concentration of the pure fractions gave an oil, lyophilization from 7% water / ...
Embodiment 3
[0225]
[0226] Compound 11 was synthesized according to the method described in Example 2, using methylhydrazine instead of hydrazine. ([M+H] = 464.7 m / z).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com