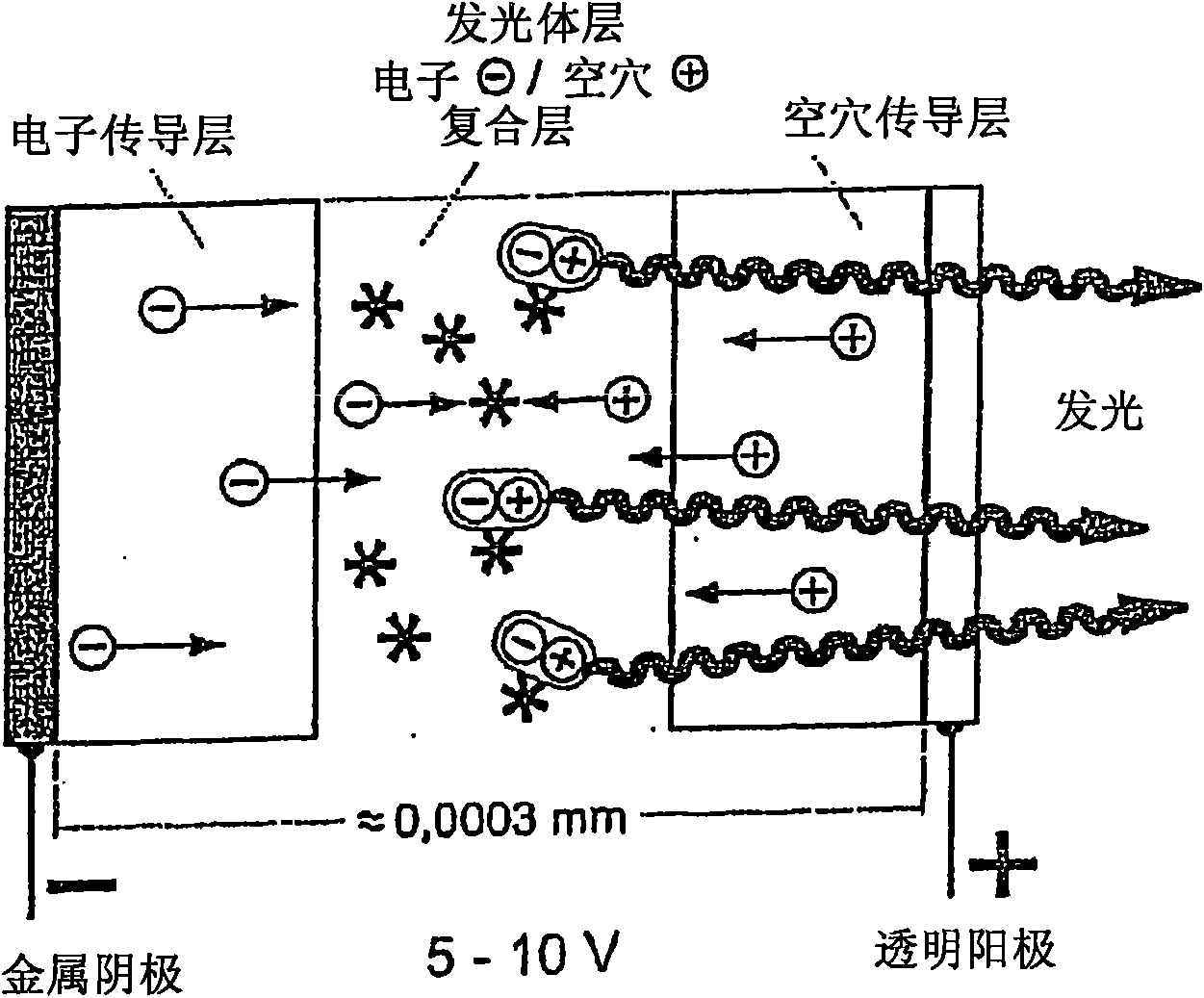
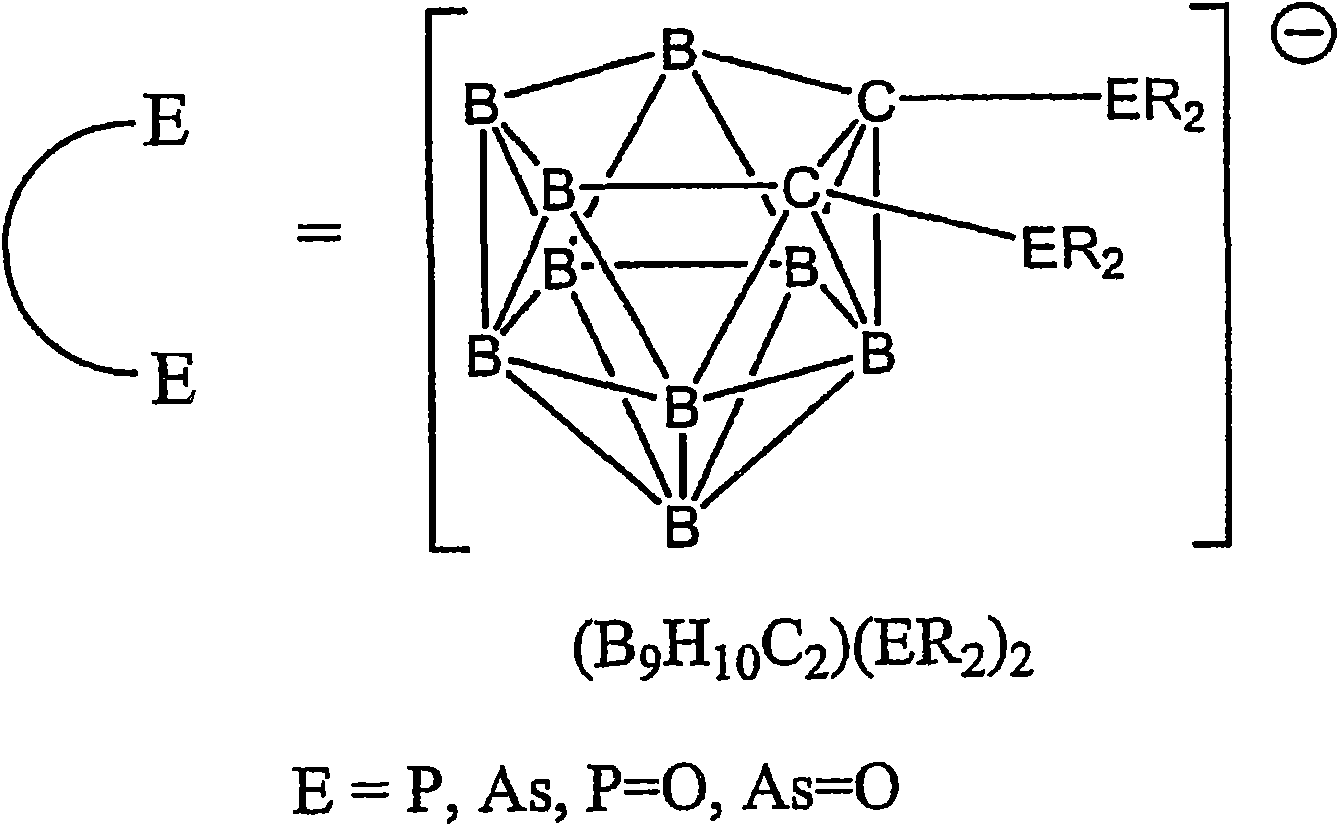
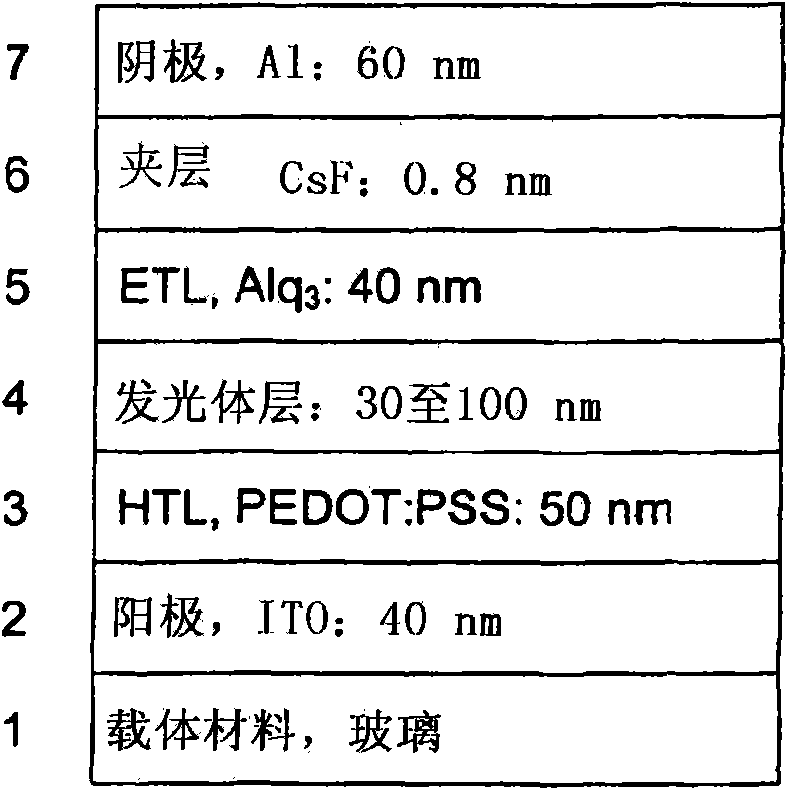
Luminescent metal complexes for organic electronic devices
A technology of organic electronic devices and metal complexes, applied in the direction of osmium organic compounds, ruthenium organic compounds, rhodium organic compounds, etc., can solve problems such as the formation of undesirable aggregates, low sublimation ability, and manufacturing reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0235] Embodiment 1: the synthesis of Pt(ppy) (7a)
[0236] The complex Pt(ppy)(7a) was prepared by the reaction of Pt(ppy)(ppyH)Cl and 1,2-bis(diphenylphosphine)-closed-carborane (Scheme 2). Equimolar amounts of Pt(ppy)(ppyH)CI and 1,2-bis(diphenylphosphine)-close-carborane were heated in ethanol at reflux under argon for 12 h. After cooling to room temperature, the crude product precipitated from the reaction mixture was purified by chromatography (AI 2 o 3 , hexane / dichloromethane). The purification is carried out by crystallization from hot ethanol.
[0237] Reaction 2
[0238] The molecular structure of Pt(ppy)(7a) in the crystal is shown in Image 6 middle. The photoluminescence spectrum of Pt(ppy)(7a) is shown in Figure 12 middle.
Embodiment 2
[0239] Embodiment 2: the synthesis of Pt(dfppy) (7a)
[0240] The complex Pt(dfppy) was prepared by the reaction of Pt(dfppy)(dfppyH)Cl and 1,2-bis(diphenylphosphine)-closed-carborane (Equation 3). Equimolar amounts of Pt(dfppy)(dfppyH)CI and 1,2-bis(diphenylphosphine)-close-carborane were stirred and refluxed in ethanol under argon for 12 h. After cooling to room temperature, the crude product precipitated from the reaction mixture was purified by chromatography (AI 2 o 3 , hexane / dichloromethane). Alternatively, the product was recrystallized from hot ethanol.
[0241] Reaction 3
[0242] The molecular structure of Pt(dfppy)(7a) in the crystal is shown in Figure 7 middle. The phosphorescence spectrum of Pt(dfppy)(7a) is shown in Figure 13 middle.
Embodiment 3
[0243] Example 3: Pt(ppy)(7b)
[0244] The reaction mixture obtained from Example 1 was chromatographed (Al 2 o 3 , hexane / dichloromethane), a small amount of Pt(ppy) (7b) was obtained. This species is less polar than the main product, Pt(ppy) (7a). The molecular structure of Pt(ppy)(7b) is shown in Figure 8 middle. The phosphorescence spectrum of Pt(ppy)(7b) is shown in Figure 14 middle.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com