Preparation method of carbamyl benzotriazole
A technology of carbamoylbenzotriazole and benzotriazole, which is applied in the field of preparation of organic compounds, can solve the problems of limited application, many steps, and few derivatives, and achieve low environmental pollution, mild reaction conditions, and rapid reaction Effect
Inactive Publication Date: 2010-04-07
ZHEJIANG NORMAL UNIVERSITY
View PDF1 Cites 2 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Christopher John Perry, Keith Holding, and Elizabeth Tyrrell Simple Novel Synthesis for 1-Carbamoyl-lHbenzotriazole and Some of Its Analogs [J]. Synth. Commun.; 2008, 38: 3354-3365. Reported the formation of amino groups from aniline through a series of reactions formylbenzotriazole, but the method has many steps and few derivatives, which limits its application in industrial production
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
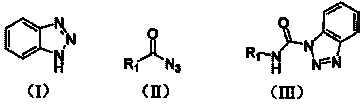
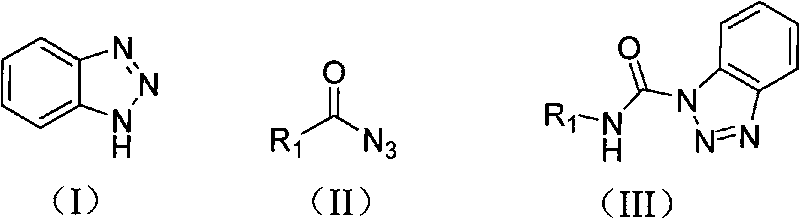
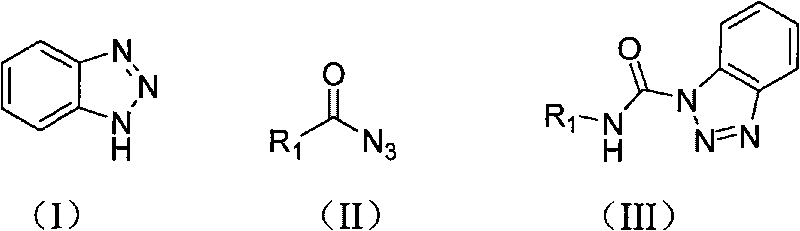
The invention relates to a preparation method of carbamyl benzotriazole, which is characterized in that benzotriazole shown in the following formula (I) serves as the raw material; in organic solvent, benzotriazole reacts with acid azide shown in the following formula (II) at the reaction temperature of 60-80 DEG C for 0.5-2 hours; after completely reacting, aftertreatment is carried out on obtained reaction mixture to obtain carbamyl benzotriazole shown in the following formula (III), and batch charging substances of acid azide and benzotriazole have the mass ratio of 1.0:1.0-1.5; R in the formulas (II) and (III) is one of the following elements: phenyl, mono-substituted phenyl, hybridization aryl, disubstituted phenyl, polysubstituted phenyl, aryl-substituted alkenyl and alkyl-substituted alkenyl. The preparation method of the invention has short reaction time, simple aftertreatment and high yield.
Description
technical field The invention relates to a preparation method of an organic compound, in particular to a preparation method of carbamoylbenzotriazole. Background technique Isocyanate is an important intermediate in organic synthesis. It is easy to react with N-, S-, O- and other nucleophiles to generate some useful chemical intermediates such as urea, thiocarbamate, carbamate, etc., which can be used in The preparation of various insecticides, fungicides, and herbicides can also be used to improve the waterproof performance of plastics, fabrics, leather, etc. It is worth pointing out that because many isocyanates are liquid, easy to decompose, and highly toxic, it is very unfavorable for storage and transportation in large-scale production. Therefore, many research groups at home and abroad are committed to finding a universally applicable isocyanate substitute. Eduardo Garci′a-Egido, Miryam Femández-Suárez, and Luis Synthesis ofCarbamoyl Azides from Primary Amines and Ca...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07D249/18C07D405/12
Inventor 王小霞钟志芸周列锦
Owner ZHEJIANG NORMAL UNIVERSITY
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Patsnap Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com