Flame-retardant lithium ion battery electrolyte
A lithium-ion battery and electrolyte technology, applied in secondary batteries, circuits, electrical components, etc., can solve problems such as fire, explosion, increased battery complexity, and production costs, and achieve improved flame retardancy and low cycle performance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] In an argon-filled glove box (H 2 4 parts of 100g lithium-ion battery electrolyte EC / EMC / DMC=1:1:1 (wt%) 1mol / L LiPF were prepared respectively in (06 , recorded as A, B, C, and D samples, and then add ethylene bis-tetrabromophthalimide with a mass percentage of 1, 3, and 5 in B, C, and D samples respectively;
[0020]
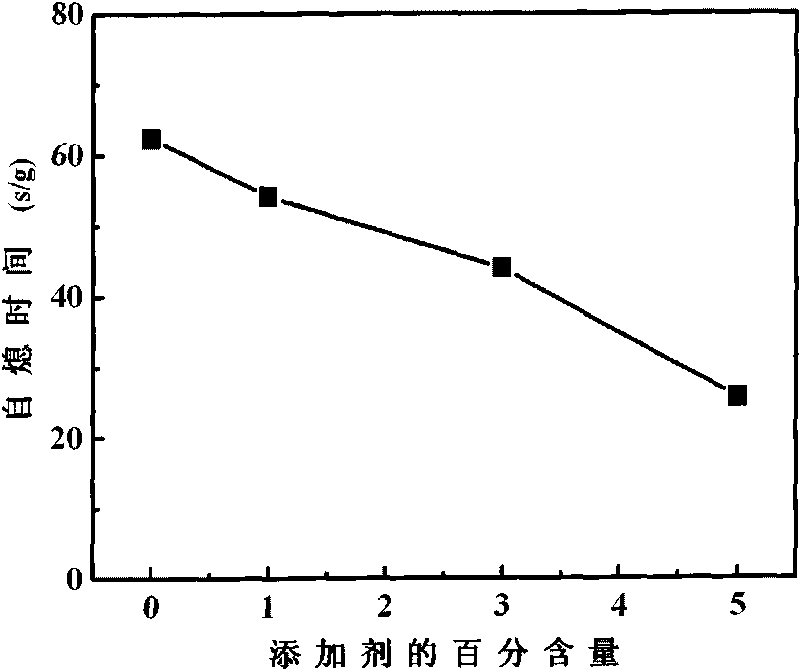
[0021] Such as figure 1 It can be seen that with the increase of the mass percentage of the additive, the self-extinguishing time decreases continuously, which proves that the flame retardancy of the ethylene bis-tetrabromophthalimide additive used in the electrolyte of lithium-ion batteries is better.
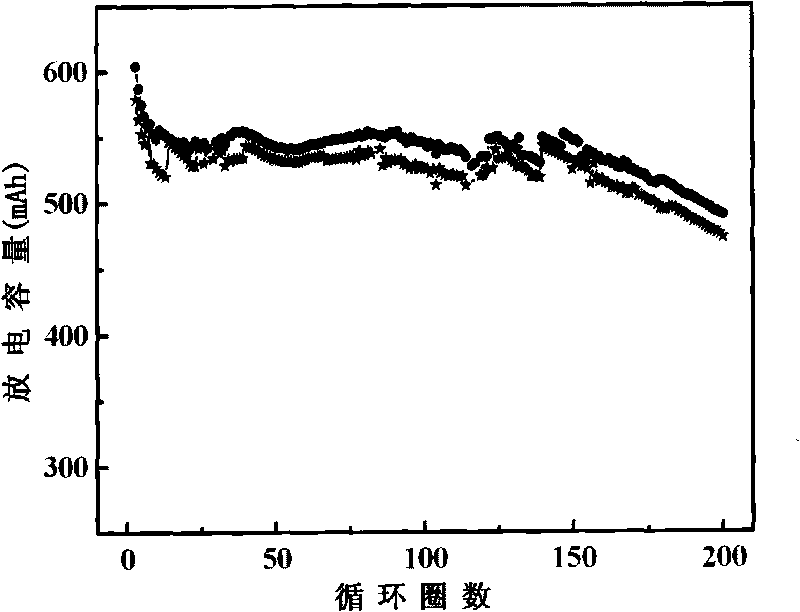
[0022] figure 2 It is the charge-discharge cycle performance curve of the aluminum shell lithium battery injected into the electrolyte A and D samples. It can be seen from the figure that the addition of additives in the lithium-ion battery electrolyte has little effect on the cycle performance and discharge capacity of the lithium battery.
Embodiment 2
[0024] In an argon-filled glove box (H 2 4 parts of 100g lithium-ion battery electrolyte EC / EMC / DMC=1:1:1 (wt%) 1mol / L LiPF were respectively configured in (06 , recorded as samples E, F, G, and H, and then adding ethylene bis-tetrachloromethylphthalimide with a mass percentage of 1, 3, and 5 to samples F, G, and H respectively;
[0025]
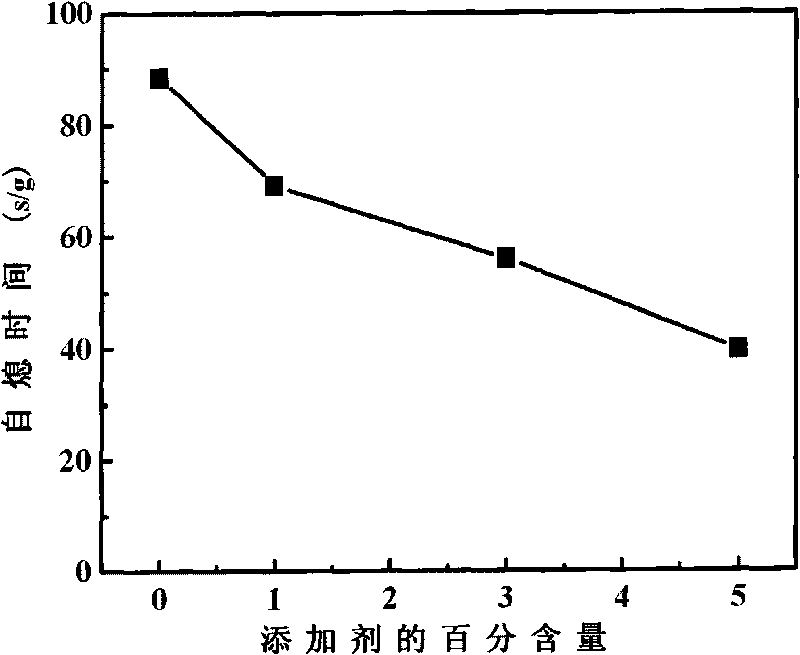
[0026] Such as image 3 It can be seen that when the addition of additives increases from 0wt% to 5wt%, the self-extinguishing time can be reduced from 88s / g to 39s / g, which proves that the ethylene bis-tetrachloromethylphthalamide used in lithium-ion battery electrolyte The flame retardancy of imide additive is better.
[0027] Figure 4 It is the charge-discharge cycle performance curve of the aluminum shell lithium battery injected with electrolyte E and H samples. It can be seen from the figure that the addition of additives in the lithium-ion battery electrolyte has almost no effect on the cycle performance and discharge capacity o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com