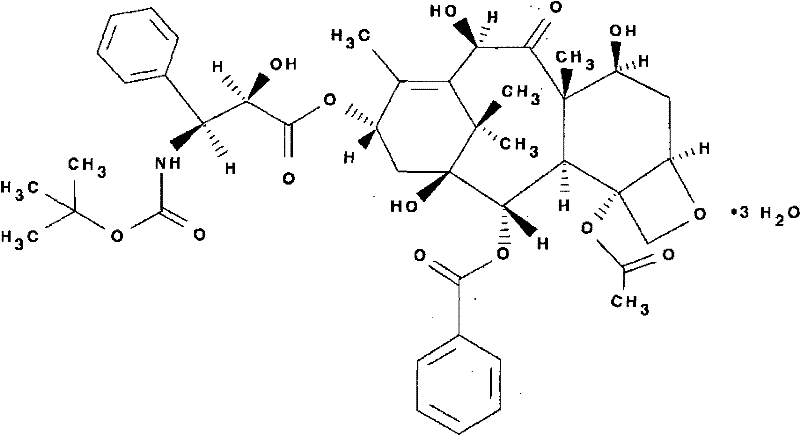
Medicine composition containing docetaxel and preparation method thereof
A docetaxel and composition technology, which is applied in the field of processing and improvement of detailed problems, can solve the problems of increasing the dissolution time of docetaxel and related additives, unfavorable stability of docetaxel injection, and difficulty in dispersion, etc. The effect of reducing the risk of foreign microbial contamination, short dissolution and dispersion time, and high dispensing accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] The refining of embodiment 1 polysorbate 80
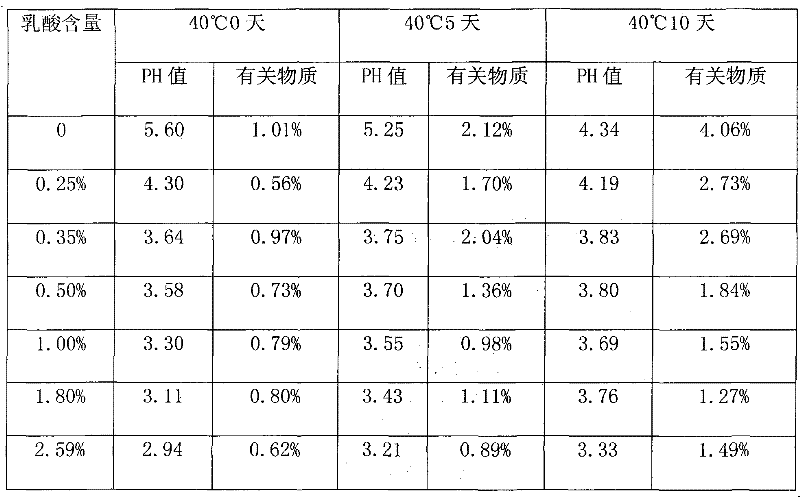
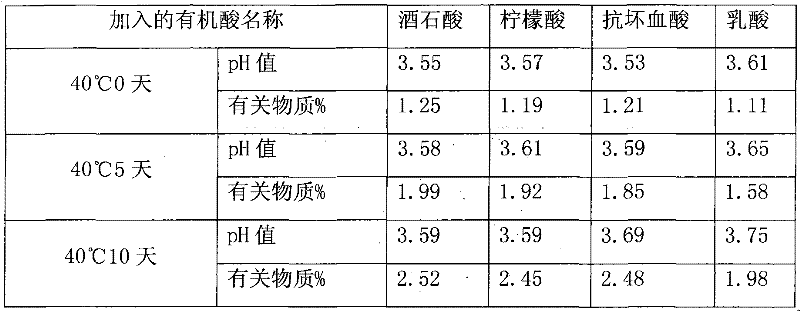
[0020] Take 750g of polysorbate 80 and add it to a 2000ml clean and dry three-neck flask equipped with an ultrasonic device and mechanical stirring, add 3.8g of lactic acid and mix evenly, adjust the pH to 3.56, add 7.5g of activated medicinal charcoal, and heat it in a water bath to 60 ℃±2℃, after stirring for 30 minutes, check the pH value to 3.63, and filter while hot with a 0.22um microporous membrane to obtain refined polysorbate 80.
Embodiment 2
[0021] Embodiment 2, the preparation of a kind of 60mg specification docetaxel injection
[0022] Put 10 grams of docetaxel in a clean and dry 1000 mL three-neck flask equipped with an ultrasonic device and mechanical stirring, add 10 grams of absolute ethanol, add 30 grams of refined polysorbate 80, and ultrasonically oscillate until the docetaxel is completely dissolved. Ultrasonic oscillation, 30-40°C, and 2KPa pressure for 30 minutes, add 300 grams of refined polysorbate 80, start mechanical stirring, nitrogen protection, continue stirring for two hours, use 0.22um microporous filter membrane to sterilize and filter, press Each bottle of 60mg of the main drug can be obtained after sub-packaging, stoppering and capping.
Embodiment 3
[0023] Embodiment 3, the preparation of a kind of 80mg specification docetaxel injection
[0024] Put 40 grams of docetaxel in a clean and dry 2000 mL three-necked flask equipped with an ultrasonic device and mechanical stirring, add 50 grams of absolute ethanol, add 60 grams of refined polysorbate 80, and ultrasonically oscillate until the docetaxel is completely dissolved. Ultrasonic oscillation, 30-40°C, and 5KPa pressure for 30 minutes, add 1200 grams of refined polysorbate 80, start mechanical stirring, nitrogen protection, continue stirring for two hours, use 0.22um microporous filter membrane to sterilize and filter, press Each bottle of 80mg of the main drug can be obtained after sub-packaging, stoppering and capping.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com