Paradoximes as HIV reverse transcriptase inhibitor as well as preparation method and purpose thereof
A technology of compounds and medicinal salts, which is applied in the field of preparation of anti-HIV drugs, can solve the problems of easy drug resistance and high frequency of daily doses, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
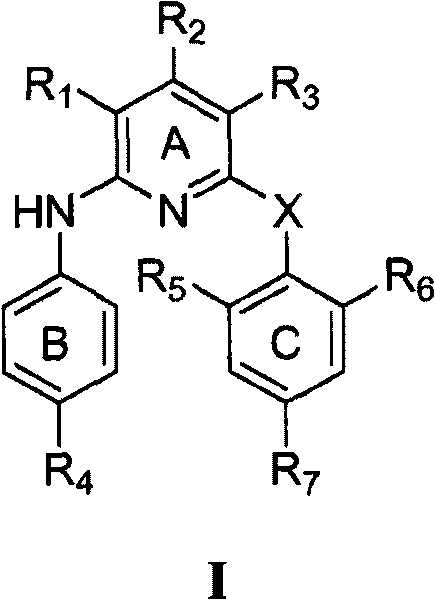
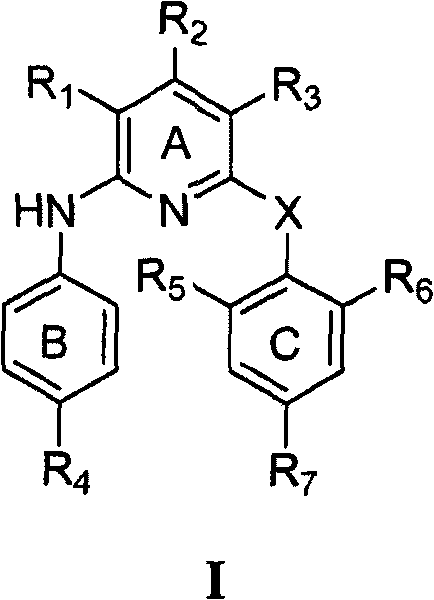
[0156] According to a preferred embodiment of the present invention, X is -O-.
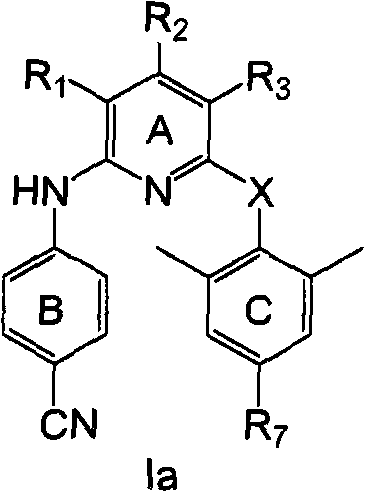
[0157] According to a preferred embodiment of the present invention, the compound of formula I of the present invention has the following formula Ia:
[0158]
[0159] or a pharmaceutically acceptable salt thereof,
[0160] in,
[0161] R 1 and R 3 each independently -NO 2 , -NH 2 , halogen, -OH, -CN or -N(R) 2 ;
[0162] R 2 for -H;
[0163] R 7 -CN, -HC=CH-CN, halogen, -CH 3 、-OCH 3 , -NH 2 、-CH 2 -NHR', -OH, -NO 2 、-CF 3 , -CH=CH 2 , -C≡CH, -C≡CR', -CH=CHR', -CH=CHCOR', -CHO, -C≡CR", -CH=CHR", -C≡C-CN, containing 1-3 A five-membered heteroaryl group of heteroatoms selected from N, O, S, and optionally with aldehyde, ketone, cyano, α, β unsaturated cyano, alkene, alkyne, aldehyde in its ring structure or a substituent of a keto group;
[0164] R' is H or C 1-6 Hydrocarbyl; R" is NO 2 , NH 2 , or N 3 ;
[0165] X is -O-, -NH-, or -NCOR-; and
[0166] R is C 1-4 Hydroc...
preparation Embodiment 1
[0257] Preparation Example 1 : the preparation of 6-chloro-2-(4-cyanoanilino)-3-nitropyridine (IV-1)
[0258] 2,6-dichloro-3-nitropyridine (II-1, 193mg, 1mmol) and p-cyanoaniline (III-1, 236mg, 2mmol) were dissolved in N,N-dimethylformamide (DMF, 3mL ). After cooling in an ice-water bath, potassium tert-butoxide (224 mg, 2 mmol) was added in batches, and then reacted at room temperature for 2 h. The reaction solution was poured into ice water, the pH value was adjusted to 5-6 with dilute HCl, and stirred for 30 minutes to precipitate a solid. The solid was filtered out, washed with water until neutral, dried, and separated on a silica gel column (dichloromethane as the eluent) to obtain compound IV-11 (186 mg, 68%) as a pale yellow solid, mp 175-178°C. 1 H NMR (CDCl 3 )δ10.47 (1H, br s, NH), 8.53 (1H, d, J=8.4Hz, ArH-4), 7.86 (2H, d, J=8.8Hz, ArH-2', 6'), 7.70 (2H, d, J=8.8Hz, ArH-3', 5'), 6.96 (1H, d, J=8.4Hz, ArH-5); MS (m / z): 275 (M + ).
preparation Embodiment 2
[0259] Preparation Example 2 : Preparation of 2-anilino-6-chloro-3-nitropyridine (IV-2)
[0260]Add 2,6-dichloro-3-nitropyridine (II-1, 193 mg, 1 mmol), aniline (III-2, 93 mg, 1 mmol) and sodium bicarbonate (84 mg, 1 mmol) into 10 mL of absolute ethanol, Reaction at room temperature for 24h. The reaction solution was poured into ice water, the pH value was adjusted to 5-6 with dilute HCl, and stirred for 30 minutes to precipitate a solid. The solid was filtered off, washed with water until neutral, and dried. Compound IV-2 (219mg, 88g) was obtained as a red solid, mp 95-98°C. 1 H NMR (CDCl 3 )δ10.28 (1H, b s, NH), 8.47 (1H, d, J=8.4Hz, ArH-4), 7.66 (2H, d, J=8.0Hz, ArH-2', 6'), 7.42( 2H, t, J=8.0Hz, ArH-3', 5'), 7.21 (1H, t, J=7.2Hz, ArH-4'), 6.81 (1H, d, J=8.4Hz, ArH-5) ; MS (m / z): 250 (M + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com