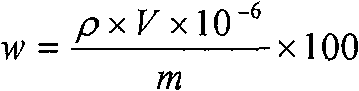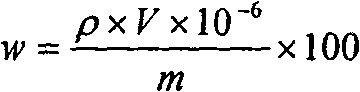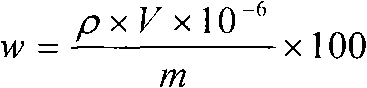Method for measuring content of boron in cobalt-base alloy
A cobalt-based alloy, boron content technology, used in the preparation of test samples, color/spectral properties measurement, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] (1.2), nitric acid, ρ1.42g / mL; superior grade;
[0042] (1.3), hydrofluoric acid, ρ1.15g / mL; superior grade;
[0043] (1.4), cobalt standard solution: 10.0mg / mL; weigh 5.000g; >99.98% metal cobalt is placed in a 250mL beaker, heated and dissolved with 15mL hydrochloric acid (1.1) at low temperature, cooled, transferred to a 500mL volumetric flask, and diluted with water to the mark, shake well;
[0044] (1.5), boron standard solution A: 1.00mg / mL; weigh 5.7192g of boric acid and dissolve it in 20mL of water, heat slightly to dissolve, transfer to a 500mL volumetric flask, dilute with water to the mark, and shake well;
[0045] (1.6), boron standard solution B: 0.10 mg / mL; pipette 25.00 mL of boron standard solution A (1.5) into a 250 mL volumetric flask, add 10 mL of nitric acid (1.2), dilute to the mark with water, and shake well;
[0046] (1.7), boron standard solution C: 0.01mg / mL; pipette 25.00mL boron standard solution B (1.6) into a 250mL volumetric flask, add 1...
Embodiment 2
[0065] (1.2), nitric acid, ρ1.42g / mL; superior grade;
[0066] (1.3), hydrofluoric acid, ρ1.15g / mL; superior grade;
[0067] (1.4), cobalt standard solution: 10.0mg / mL; weigh 5.000g; >99.98% metal cobalt is placed in a 250mL beaker, heat and dissolve with 5-15mL hydrochloric acid (1.1) at low temperature, cool, transfer to a 500mL volumetric flask, Dilute to the mark with water and shake well;
[0068] (1.5), boron standard solution A: 1.00mg / mL; weigh 5.7192g of boric acid and dissolve it in 20mL of water, heat slightly to dissolve, transfer to a 500mL volumetric flask, dilute with water to the mark, and shake well;
[0069] (1.6), boron standard solution B: 0.10 mg / mL; pipette 25.00 mL of boron standard solution A (1.5) into a 250 mL volumetric flask, add 10 mL of nitric acid (1.2), dilute to the mark with water, and shake well;
[0070] (1.7), boron standard solution C: 0.01mg / mL; pipette 25.00mL boron standard solution B (1.6) into a 250mL volumetric flask, add 10mL nitr...
Embodiment 3
[0088] (1.2), nitric acid, ρ1.42g / mL; superior grade;
[0089] (1.3), hydrofluoric acid, ρ1.15g / mL; superior grade;
[0090] (1.4), cobalt standard solution: 10.0mg / mL; weigh 5.000g; >99.98% metal cobalt is placed in a 250mL beaker, heated and dissolved with 15mL hydrochloric acid (1.1) at low temperature, cooled, transferred to a 500mL volumetric flask, and diluted with water to the mark, shake well;
[0091] (1.5), boron standard solution A: 1.00mg / mL; weigh 5.7192g of boric acid and dissolve it in 20mL of water, heat slightly to dissolve, transfer to a 500mL volumetric flask, dilute with water to the mark, and shake well;
[0092] (1.6), boron standard solution B: 0.10 mg / mL; pipette 25.00 mL of boron standard solution A (1.5) into a 250 mL volumetric flask, add 10 mL of nitric acid (1.2), dilute to the mark with water, and shake well;
[0093] (1.7), boron standard solution C: 0.01mg / mL; pipette 25.00mL boron standard solution B (1.6) into a 250mL volumetric flask, add 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com