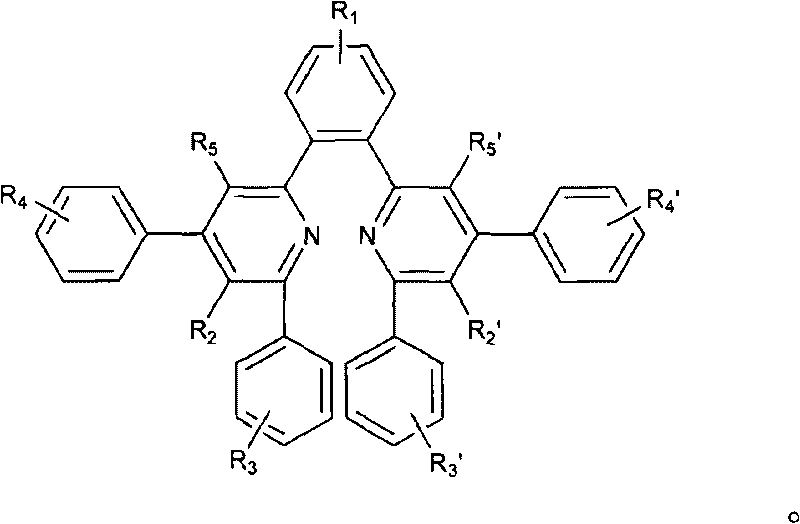
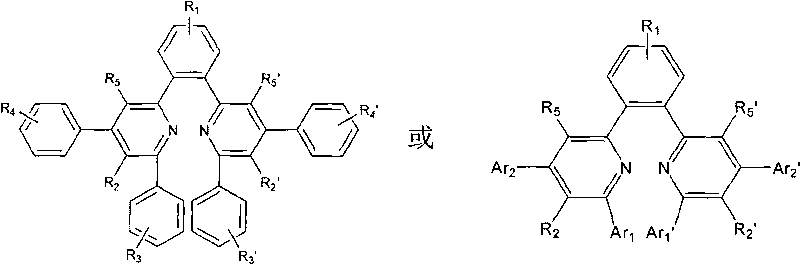
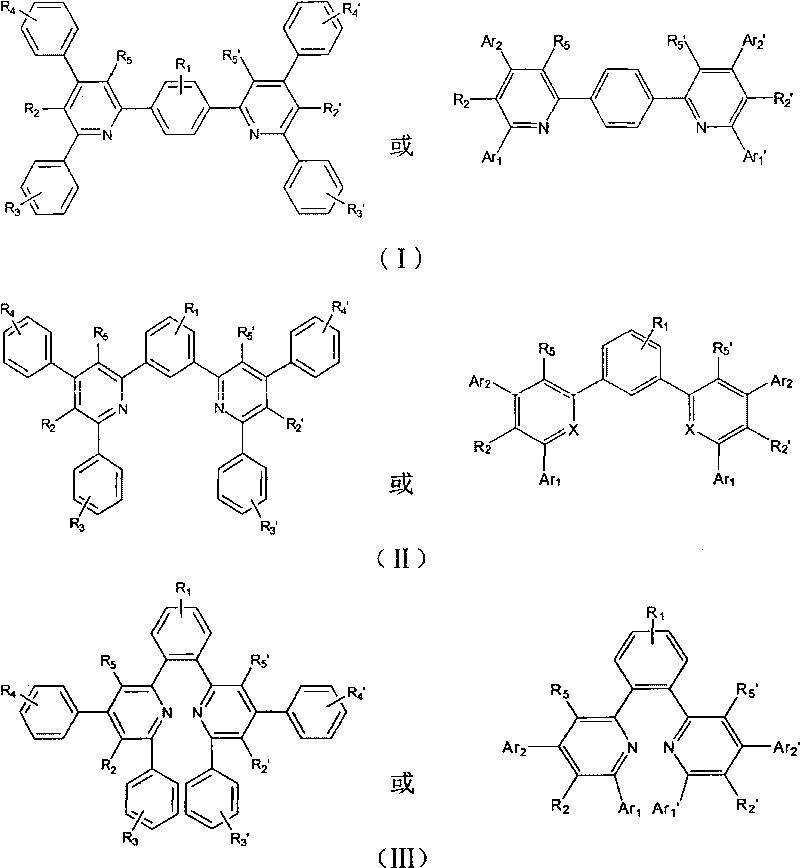
Organic electronic transmission and/or hole barrier materials, synthesis method and use thereof
A hole-blocking material and organic electron technology, applied in chemical instruments and methods, light-emitting materials, organic chemistry, etc., can solve the problems of changes in light-emitting properties, further improvement of stability, and poor device performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11
[0056] Embodiment 1.1, the preparation of 4-bis(2-(4,6-diphenyl-5-trifluoromethylpyridine))benzene (BDTPB)
[0057]
[0058] The first step: take 2-bromo-1-(4-trifluoromethylphenyl)ethanone and pyridine with a molar ratio of 1 as raw materials, stir at room temperature for 10 hours, filter, and wash with a large amount of water to obtain the corresponding pyridine Bromide, the yield is about 90%;
[0059] The second step: under the condition of nitrogen protection, add the product of the first step, p-benzophenone and benzaldehyde (2:1:2 in molar ratio) into the three-necked bottle, and then add appropriate amount of glacial acetic acid and ammonium acetate , stirred vigorously, kept the temperature at 120° C. to 140° C., refluxed for 24 hours, filtered out the product, and subjected to column chromatography or recrystallization to obtain the high-purity target product with a yield of about 50%.
[0060] m / z: 672.20 (100.0%), 673.20 (46.2%), 674.21 (10.2%), 675.21 (1.5%). ...
Embodiment 21
[0070] Example 2.1, Preparation of 4-bis(2-(6-phenyl-4-p-tolyl-5-trifluoromethylpyridine))benzene (BPTTPB)
[0071]
[0072] The first step: take 2-bromo-1-(4-trifluoromethylphenyl)ethanone and pyridine with a molar ratio of 1 as raw materials, stir at room temperature for 8 hours, filter, and wash with a large amount of water to obtain the corresponding pyridine Bromide, the yield is about 90%;
[0073] The second step: under the condition of nitrogen protection, the product of the first step, p-benzenediphenone and p-tolualdehyde (2:1:2 in molar ratio) are added in the three-necked flask, and then an appropriate amount of glacial acetic acid is added and ammonium acetate, stirred vigorously, kept the temperature at 120°C to 140°C, refluxed for 24 hours, filtered out the product, and subjected to column chromatography or recrystallization to obtain the high-purity target product with a yield of about 55%.
[0074] m / z: 700.23 (100.0%), 701.23 (48.3%), 702.24 (11.2%), 703....
Embodiment 36
[0075] Embodiment 3.6, the preparation of 6'-(1,4-phenylene) bis(2,4-diphenylpyridinenitrile) (PBDNN)
[0076]
[0077]The first step: take 4-(2-bromoacetyl) benzonitrile and pyridine with a molar ratio of 1 as raw materials, stir at room temperature for 7 hours, filter, and wash with a large amount of water to obtain the corresponding pyridinium bromide salt with a yield of about 85%;
[0078] The second step: under the condition of nitrogen protection, add the product of the first step, p-benzophenone and benzaldehyde (2:1:2 in molar ratio) into the three-necked bottle, and then add appropriate amount of glacial acetic acid and ammonium acetate , stirred vigorously, kept the temperature at 120° C. to 140° C., refluxed for 24 hours, filtered out the product, and subjected to column chromatography or recrystallization to obtain the high-purity target product with a yield of about 60%.
[0079] m / z: 586.22 (100.0%), 587.22 (45.7%), 588.22 (10.7%), 589.23 (1.5%), 587.21 (1.5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com