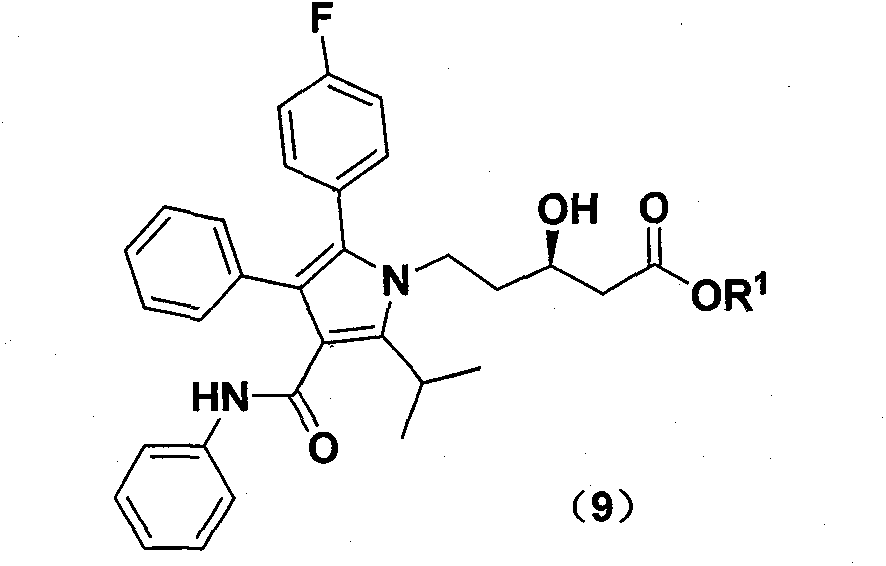
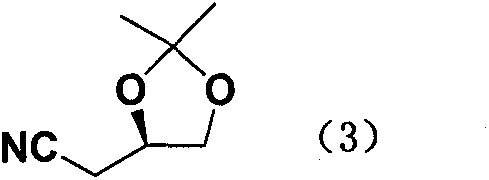
New preparation method of atorvastatin calcium 1H-pyrrole derivatives
A technology of pyrrole and compound, which is applied in the field of chemical pharmaceuticals and can solve problems such as high prices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Add compound 1221.0g (2.0mol), NaCN 113.5g (2.2mol), 550ml DMF, 75ml CH 3 OH, the temperature was raised to 60°C to react, the massive solid in the system gradually disappeared and a white powdery solid was formed. After reacting for 8h, it was filtered and the solid was treated with NaClO solution. The mother liquor is concentrated by distillation under reduced pressure (distillation temperature does not exceed 100°C), and the solvent in the system is evaporated to dryness as much as possible. The evaporated solvent can be recycled and used mechanically. Add 40ml of concentrated HCl to adjust the pH of the system to 1-2, and then add 200-250ml acetone, stirred, filtered, the solid was treated with NaClO solution, and the mother liquor was collected and concentrated. Distilled under reduced pressure and collected (T=130°C) fractions to obtain a yellow oily liquid. Yield: 55%
Embodiment 2
[0050] Add compound 1221.0g (2.0mol), NaCN 113.5g (2.2mol), 500ml acetonitrile, 70ml CH 3 OH, the temperature was raised to 60°C to react, the massive solid in the system gradually disappeared and a white powdery solid was formed. After reacting for 10 h, it was filtered and the solid was treated with NaClO solution. The mother liquor is concentrated by distillation under reduced pressure (distillation temperature does not exceed 100°C), and the solvent in the system is evaporated to dryness as much as possible. The evaporated solvent can be recycled and used mechanically. Add 40ml of concentrated HCl to adjust the pH of the system to 1-2, and then add 200-250ml acetone, stirred, filtered, the solid was treated with NaClO solution, and the mother liquor was collected and concentrated. Distilled under reduced pressure and collected (T=130°C) fractions to obtain a yellow oily liquid. Yield: 45%.
Embodiment 3
[0052] Compound 1221.0g (2.0mol), KCN143g (2.2mol), 600ml DMF were added to a 1L three-neck flask, and the temperature was raised to 60°C for reaction. The massive solid in the system gradually disappeared and a white powdery solid was formed. After reacting for 10 h, it was filtered and the solid was treated with NaClO solution. The mother liquor was concentrated by distillation under reduced pressure (distillation temperature did not exceed 100°C), and the solvent in the system was evaporated to dryness as much as possible. The evaporated solvent can be recycled and used mechanically. Carefully add 40ml of concentrated HCl to adjust the pH of the system to 1-2, and then add 200 - 250ml of acetone, stirred, filtered, the solid was carefully treated with NaClO solution, the mother liquor was collected and concentrated. Distilled under reduced pressure and collected (T=130°C) fractions to obtain a yellow oily liquid. Yield: 50%.
[0053] Step B
[0054]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com