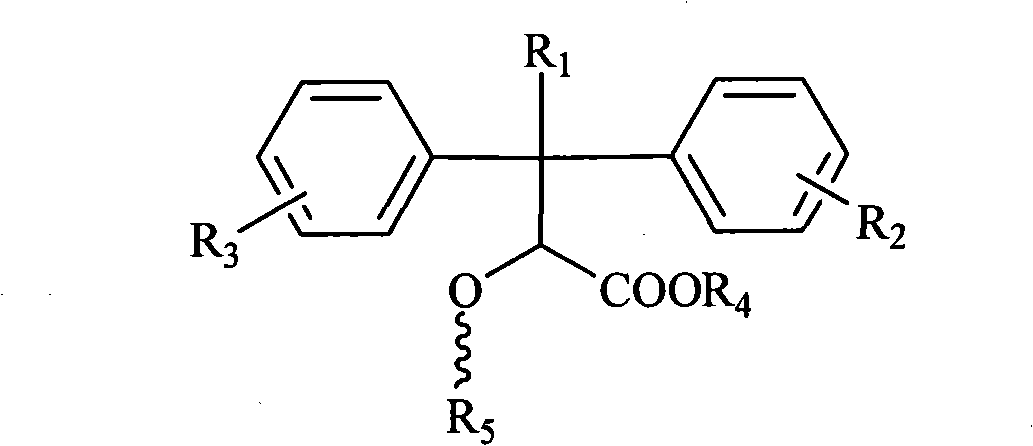
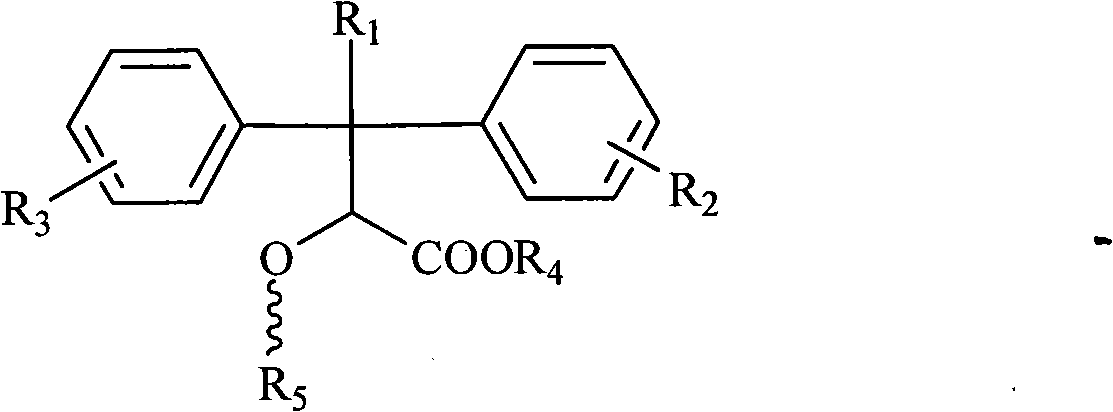
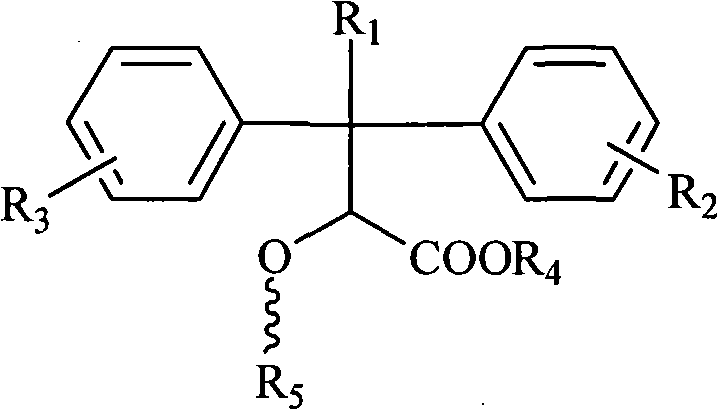
Pyridine ring-substituted alpha-hydroxycarboxylic acid derivatives, preparation method thereof and use thereof
A substituent, nitropyridine technology, applied in the application field of α-hydroxycarboxylic acid derivative corticosteroid selective endothelin receptor antagonist, preparation of antihypertensive drugs, can solve the problems of untimely treatment and lack of pulmonary arterial hypertension medications, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 13
[0070] Synthesis of embodiment 13-phenyl-3-(3-fluorophenyl)-2,3-epoxypropionic acid methyl ester
[0071]
[0072] Dissolve 42ml of 3-fluoro-benzophenone (29.646g, 0.148mmol, a type of VII) and methyl chloroacetate (a type of VI) in 40ml THF, and put it into a constant pressure funnel for use. Add 200ml THF to a 250ml three-neck flask, add sodium methoxide (24g, 0.444mmol) while stirring, cool to 0°C in an ice-salt bath, and add the above mixed solution dropwise, controlling the temperature not to exceed 10°C. After the addition, react at room temperature for 0.5 hours, add 150ml of water, extract with ethyl acetate (100ml×3), combine the organic phases, wash twice with saturated brine, dry with anhydrous sodium sulfate, filter, and distill under reduced pressure, the concentrate is 41.9g oily (a kind of V), yield 75%, ESI-MS: m / z 213[M-59], 241[M-31], 273[M+1], 295[M+23] are directly used in the following step response.
Embodiment 2-12
[0074] With reference to the synthetic operation of Example 1, different benzophenone derivatives were used to replace 3-fluoro-benzophenone (VII) in Example 1 to obtain different 3,3-diaryl-substituted-2,3 - Derivatives (V) of methyl glycidate.
[0075]
[0076]
Embodiment 163
[0077] Synthesis of Example 163-phenyl-3-(3-fluoro-phenyl)-2-hydroxyl-3-methoxypropionic acid methyl ester
[0078]
[0079] Dilute the product of Example 1 (a type of V) with 200ml of methanol (a type of IV), add p-benzenemethanesulfonic acid (0.65g, 3.42mmol) at room temperature, heat to reflux for 1h, concentrate under reduced pressure, and the residue dissolves In dichloromethane, washed with dilute sodium carbonate solution, the organic phase was dried and concentrated to give a light yellow viscous liquid as 3-phenyl-3-(3-fluoro-phenyl)-2-hydroxy-3-methoxy Methyl propionate (one of compound III), yield 80%, ESI-MS: m / z 245[M-59], 273[M-31], 305[M+1], 327[M+23 ].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com