Nimesulide dry suspension and preparation method thereof
A dry suspension and sweetener technology, which is applied in anti-inflammatory agents, non-central analgesics, active ingredients of amides, etc., can solve problems affecting clinical application, inaccurate dosage, and inconvenient administration of divided doses, etc. To achieve the effect of convenient administration in divided doses, convenient administration and accurate dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Nimesulide 100g
[0030] Hypromellose 200g
[0031] Carboxymethyl Starch Sodium 100g
[0032] Stevia 8g
[0033] Flavor 2g
[0034] Micronized silica gel 50g
[0035] Mannitol 1000g
[0036]
[0037] Make 1000 bags
[0038] Preparation
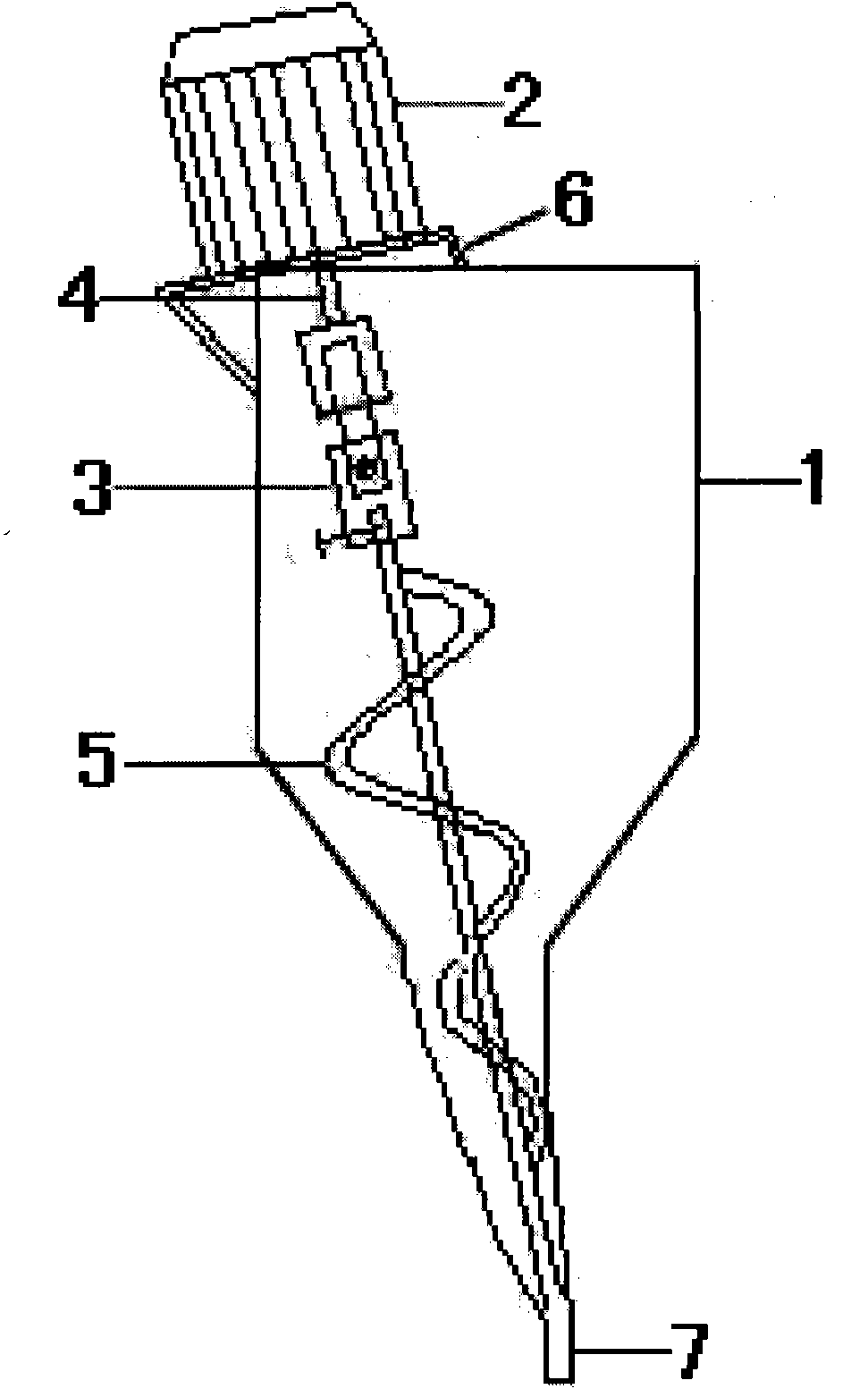
[0039] Weigh Nimesulide, Hypromellose, Carboxymethyl Starch Sodium, Stevioside, Sweetener, Essence, Micropowder Silica Gel, Mannitol respectively according to the prescription ratio, mix them evenly, grind them, pass through a 30-40 mesh sieve, mix Evenly, put it in a new type of powder feeder, heat seal it with a double-packed heat sealer for powder packaging, and make a nimesulide dry suspension packaged in a double-packed bag.
Embodiment 2
[0041] Nimesulide 50g
[0042] Hypromellose 200g
[0043] Carboxymethyl Starch Sodium 100g
[0044] Stevia 8g
[0045] Flavor 2g
[0046] Micronized silica gel 50g
[0047] Mannitol 1000g
[0048]
[0049] Make 1000 bags
[0050] Preparation
[0051] Weigh Nimesulide, Hypromellose, Carboxymethyl Starch Sodium, Stevioside, Sweetener, Essence, Micropowder Silica Gel, Mannitol respectively according to the prescription ratio, mix them evenly, grind them, pass through a 30-40 mesh sieve, mix Evenly, put it in a new type of powder feeder, heat seal it with a double-packed heat sealer for powder packaging, and make a nimesulide dry suspension packaged in a double-packed bag.
Embodiment 3
[0053] Nimesulide 25g
[0054] Hypromellose 200g
[0055] Carboxymethyl Starch Sodium 100g
[0056] Stevia 8g
[0057] Flavor 2g
[0058] Micronized silica gel 50g
[0059] Mannitol 1000g
[0060]
[0061] Make 1000 bags
[0062] Preparation
[0063] Weigh Nimesulide, Hypromellose, Carboxymethyl Starch Sodium, Stevioside, Sweetener, Essence, Micropowder Silica Gel, Mannitol respectively according to the prescription ratio, mix them evenly, grind them, pass through a 30-40 mesh sieve, mix Evenly, put it in a new type of powder feeder, heat seal it with a double-packed heat sealer for powder packaging, and make a nimesulide dry suspension packaged in a double-packed bag.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com