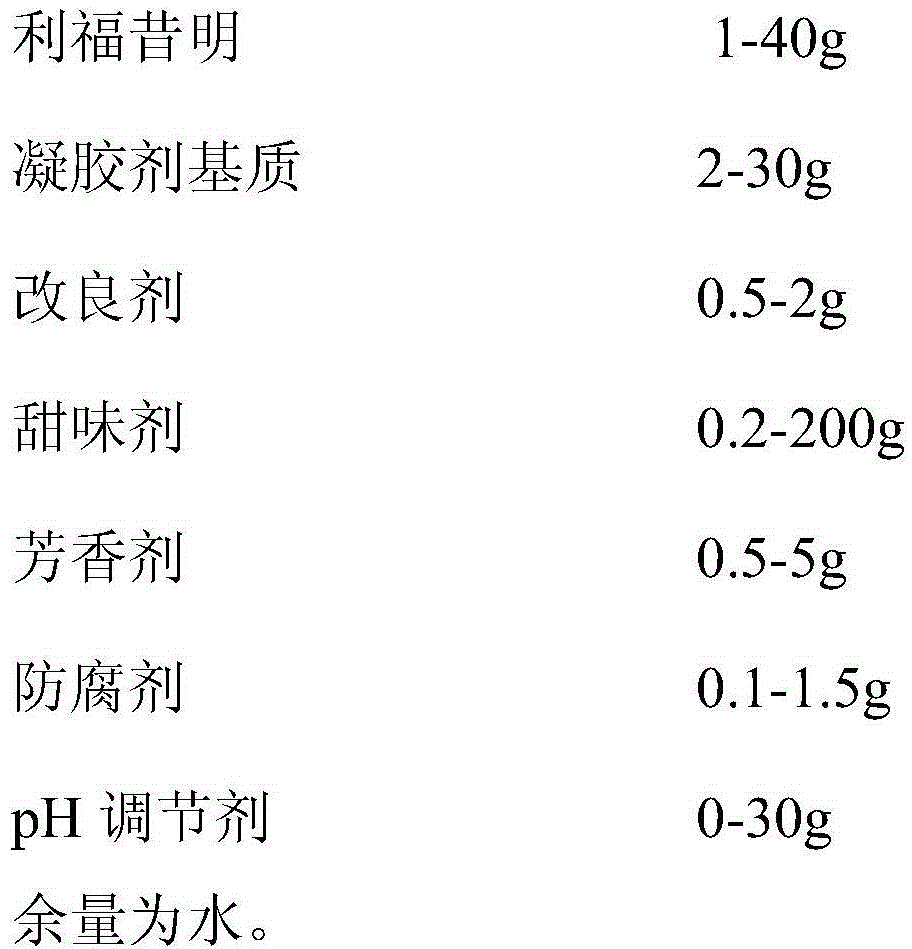
A pharmaceutical preparation containing rifaximin
A rifaximin and a rifaximin technology are applied in the field of rifaximin-containing pharmaceutical preparations for treating intestinal infections and their preparation fields, and can solve the problems of the tablet being unfavorable for children to take, inconvenient to take when going out, and unfavorable for children to take. , to reduce the fear of taking medicine, improve bioavailability and good taste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] A rifaximin gel, measured in 1000ml, consists of the following components:
[0057]
[0058] The preparation method is to add aspartame and calcium chloride to an appropriate amount of purified water to dissolve; add konjac gum and carrageenan, add purified water to 80% of the total amount, heat and stir until the sol is boiled; when the above gel is cooled to 60 ° C , add the prescribed amount of rifaximin dispersed with purified water and orange essence, potassium sorbate and citric acid dissolved in an appropriate amount of purified water, add water to the full amount, and stir well; filter while it is hot, and take the filtered liquid colloid for Subpackage, that is.
Embodiment 2
[0060] A rifaximin gel, measured in 1000ml, consists of the following components:
[0061]
[0062]
[0063] The preparation method is to dissolve steviol glycoside and potassium chloride in appropriate amount of water respectively; add carrageenan, add purified water to 80% of the total amount, heat and stir until the sol is boiled; when the above gel is cooled to 60°C, add purified water to disperse A good prescription amount of rifaximin and ethyl p-hydroxybenzoate, pineapple essence, sodium dihydrogen phosphate and disodium hydrogen phosphate dissolved in an appropriate amount of purified water, add water to the full amount, stir well, filter while hot, and take the filtered The liquid colloid is subpackaged, and it is obtained.
Embodiment 3
[0065] A rifaximin gel, measured in 1000ml, consists of the following components:
[0066]
[0067]
[0068] Preparation method: add sucrose and calcium chloride to appropriate amount of water to dissolve respectively; add agar, add purified water to 80% of the total amount, heat and stir until the sol is boiled; when the above gel is cooled to 60°C, add purified water dispersed The prescribed amount of rifaximin, sorbic acid, apple essence, citric acid, and sodium citrate dissolved in an appropriate amount of purified water, add water to the full amount, stir well, filter while it is hot, and take the filtered liquid colloid for sub-packaging to obtain .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com