Oral dispersion domperidone coutrolled-release gel and its preparing method
A technology of dipperidone gel and domperidone, applied in medical preparations containing active ingredients, organic active ingredients, pharmaceutical formulas, etc., can solve problems such as multiple side effects, low bioavailability, and inability to resist gastric acid, and achieve Accurate dosage, stable blood drug concentration, and excellent stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
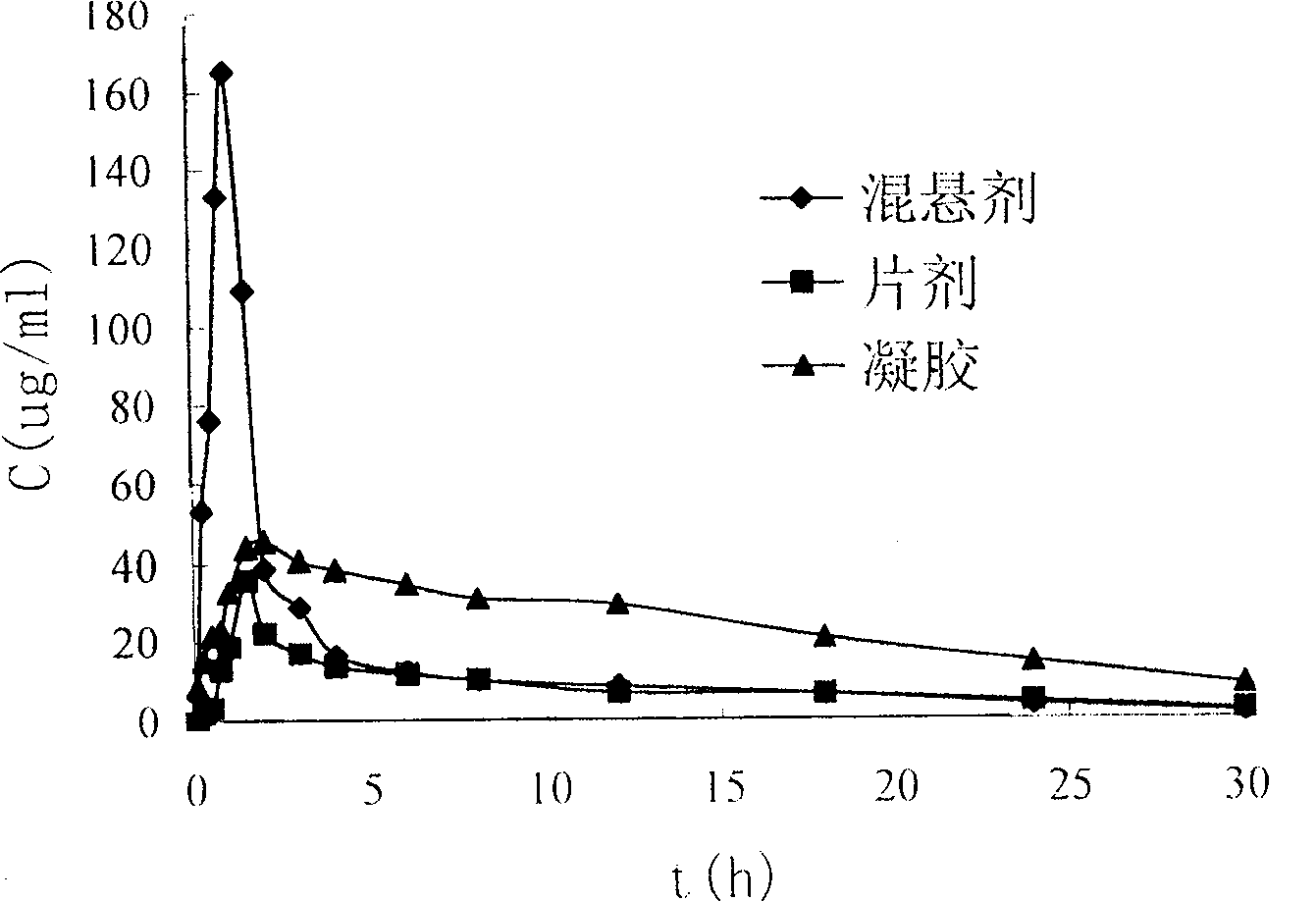
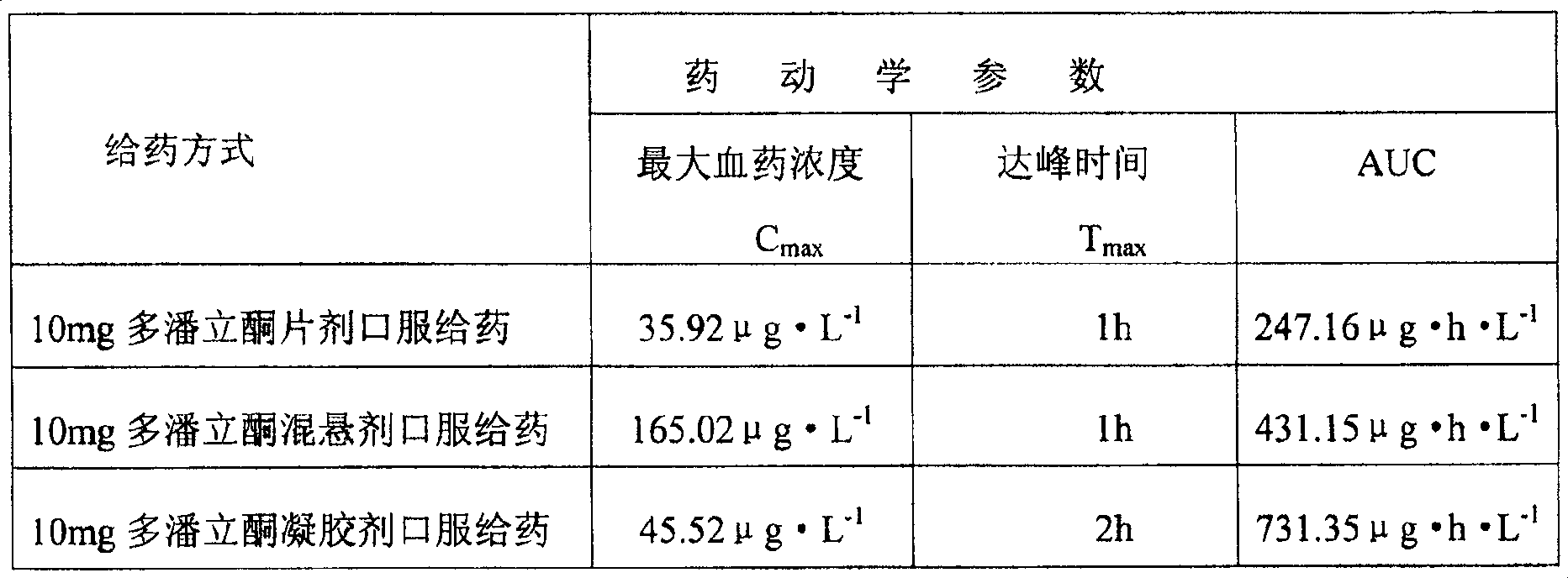
Image
Examples
Embodiment 1
[0038] Weigh 0.1g of Carbomer-P934, sprinkle it into 25ml of distilled water, and let it swell for 24 hours. Weigh 500mg of domperidone and 5g of PVPk30, mix the two, add 100ml of absolute ethanol, and place in a 50°C water bath to dissolve completely. Distill under reduced pressure in a 50°C water bath to prepare a solid dispersion. Weigh 550 mg of the prepared solid dispersion, add it into the above-mentioned swollen liquid, and stir evenly. Add 2ml of 50% triethanolamine solution to adjust the pH value to 9. Then add distilled water to 50g.
Embodiment 2
[0040]Weigh 0.1g of Carbomer-P971, sprinkle it into 25ml of distilled water, and let it swell for 24 hours. Accurately weigh 500mg of domperidone and 5g of PVPk30, mix the two, add 100ml of absolute ethanol, and place in a 50°C water bath to dissolve completely. Distill under reduced pressure in a 50°C water bath to prepare a solid dispersion. Weigh 550 mg of the prepared solid dispersion, add it into the above-mentioned swollen liquid, and stir evenly. Add 2ml of 50% triethanolamine solution to adjust the pH value to 7. Then add distilled water to 50g.
Embodiment 3
[0042] Weigh 0.1g of Carbomer-P974, sprinkle it into 25ml of distilled water, and let it swell for 24 hours. Weigh 500mg of domperidone and 5g of PVPk25, mix the two, add 100ml of absolute ethanol, place in a 50°C water bath to dissolve completely. Distill under reduced pressure in a 50°C water bath to prepare a solid dispersion. Accurately weigh 550 mg of the prepared solid dispersion, add it into the above-mentioned swollen liquid, and stir evenly. Add 2ml of 50% triethanolamine solution to adjust the pH value to 9. Then add distilled water to 50g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com