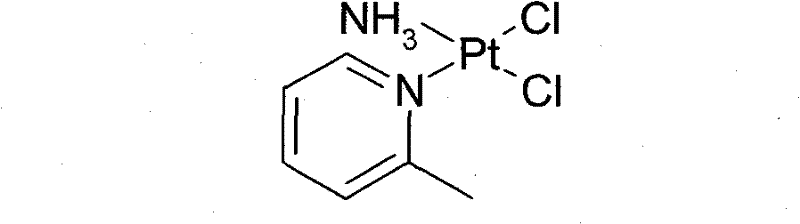
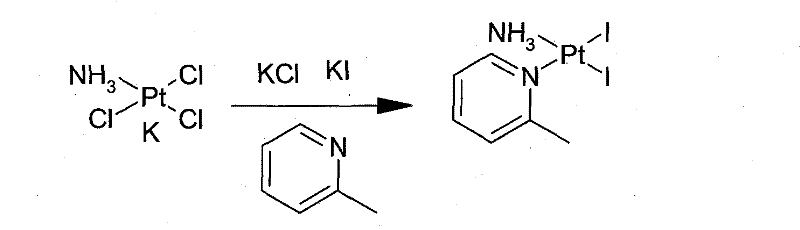
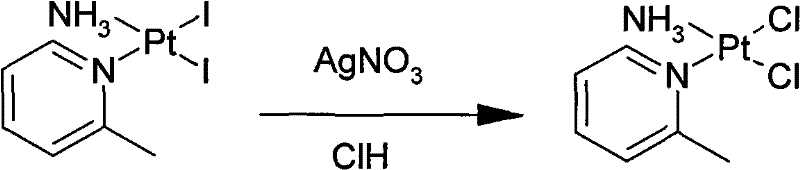
New method for preparing picoplatin
A technology of picoplatin and picoline, applied in the field of preparation of anti-tumor drug picoplatin, to achieve the effect of convenient industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] This example illustrates the preparation of picoplatin.
[0052] In the reaction flask, add K under dark conditions 2 PtCl 4 (103.8g, 0.25mol), 1500mL of deionized water, and 500mL of an aqueous solution in which KI (166g, 1mol) was dissolved was added, and the mixture was stirred at 25°C for 2 hours. 2-Methylpyridine (23.3 g, 0.25 mol) was slowly added dropwise, and the mixture was stirred at 25°C for 8 hours. Filtrate and dry under reduced pressure to obtain potassium triiodo(2-picoline)platinate(II) potassium (170.0 g, 0.24 mol) in the form of brown crystalline powder, with a yield of 96%, and keep it sealed and protected from light for future use;
[0053] Potassium triiodo (2-picoline) platinum (II) (170.0 g, 0.24 mol), 25% ammonia (16.3 g, 0.24 mol), and 2000 mL of deionized water were stirred at room temperature and protected from light. Mix evenly, stir at 25°C for 8 hours, filter, wash the filter cake with pure water, and dry under vacuum at 45°C to obtain c...
Embodiment 2
[0056] The preparation method of the picoplatin of this embodiment is the same as that of Example 1, but the reaction conditions are changed.
[0057] In the reaction flask, add K under dark conditions 2 PtCl 4 (103.8g, 0.25mol), 1500mL of deionized water, and 500mL of an aqueous solution in which KI (166g, 1mol) was dissolved was added, and the mixture was stirred at 30°C for 2 hours. 2-Methylpyridine (23.3 g, 0.25 mol) was slowly added dropwise, and the mixture was stirred at 30° C. for 8 hours. Filtrate and dry under reduced pressure to obtain potassium triiodo(2-picoline)platinate(II) potassium (163.0 g, 0.23 mol) in the form of brown crystalline powder, with a yield of 92%, and store it in an airtight and dark place for future use;
[0058] Potassium triiodo (2-picoline) platinum (II) (163.0 g, 0.23 mol), 25% ammonia (15.6 g, 0.23 mol), and 2000 mL of deionized water were stirred at room temperature and protected from light. Mix well, stir at 30°C for 8 hours, filter, ...
Embodiment 3
[0061] The preparation method of the picoplatin of this embodiment is the same as that of Example 1, but the reaction conditions are changed.
[0062] In the reaction flask, add K under dark conditions 2 PtCl 4 (103.8g, 0.25mol), 1500mL of deionized water, and 500mL of an aqueous solution in which KI (166g, 1mol) was dissolved was added, and the mixture was stirred at 15°C for 1 hour. 2-Methylpyridine (23.3 g, 0.25 mol) was slowly added dropwise, and the mixture was stirred at 15° C. for 8 hours. Filter and dry under reduced pressure to obtain potassium triiodo(2-picoline)platinate(II) potassium (172.0 g, 0.24 mol) in the form of brown crystalline powder, with a yield of 97%, and store it in a sealed and dark place for future use;
[0063] Potassium triiodo (2-picoline) platinum (II) (172.0 g, 0.24 mol), 25% ammonia (16.3 g, 0.24 mol), and 2000 mL of deionized water were stirred at room temperature and protected from light. Mix evenly, stir at 15°C for 8 hours, filter, wash...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com