Preparation of cyanocobalamine and adenosine composition and preparation method thereof
A technology of adenosylcobalamin and pharmaceutical preparations, which is applied in the field of pharmaceutical preparations to achieve the effects of low production cost, rapid drug effect, and convenient carrying and taking
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Made into 1000 pieces / grain
[0042] Preparation Process
[0043] Remarks: The following operations were all carried out under dark conditions.
[0044] a. take by weighing adenosylcobalamin, adenosine of prescription quantity, cross 80 mesh sieves,
[0045] b. Weigh the prescription amount of auxiliary materials and mix evenly, pass through an 80 mesh sieve and mix evenly.
[0046] c. Fully mix the excipients with adenosylcobalamin and adenosine in an incremental dilution method,
[0047] d. Granulating the mixture obtained in "c",
[0048] e. Press the granules obtained in "d" into tablets, and coat with a film to obtain a combined tablet of adenosylcobalamin and adenosine
[0049] Or compress the mixture obtained in "c" by direct powder compression method to obtain a combined tablet of adenosylcobalamin and adenosine
[0050] Or directly fill the granules obtained in "d" into hollow capsules to obtain a combination capsule of adenosylcobalamin and adenosine. ...
Embodiment 2
up to 1000ml
[0054] Made 1000 pieces
[0055] Preparation Process
[0056] Remarks: The following operations were all carried out under dark conditions.
[0057] a. Accurately weigh adenosylcobalamin and adenosine according to the prescription, add 70% to 90% of the prescribed amount of water for injection, stir until completely dissolved, add water for injection to the full amount, and control the pH value at 3.5-7.5;
[0058] b. Add 0.01% to 0.20% activated carbon to the solution obtained in "a", stir at 10°C to 60°C for 5min to 40min, filter for decarburization, and then filter through a 0.22μm microporous membrane until clear;
[0059] c. Measure the pH value and content of the solution obtained in "b". After passing the test, it is divided into 1000 ampoules, sealed, and sterilized by circulating steam at 100°C for 15 minutes to obtain a combined injection of adenosylcobalamin and adenosine, J , K, L.
[0060] d. Add 0.01% to 0.20% activated carbon to the so...
Embodiment 3
[0064] Made 1000 pieces
[0065] Preparation Process
[0066] Remarks: The following operations were all carried out under dark conditions.
[0067] a. Accurately weigh adenosylcobalamin, adenosine and auxiliary materials according to the prescription, add 70% to 90% of the prescribed amount of water for injection, stir until completely dissolved, add water for injection to the full amount, and control the pH value at 3.5-7.5;
[0068] b. Add 0.01% to 0.20% activated carbon to the solution obtained in "a", stir at 10°C to 60°C for 5min to 40min, filter for decarburization, and then filter through a 0.22μm microporous membrane until clear;
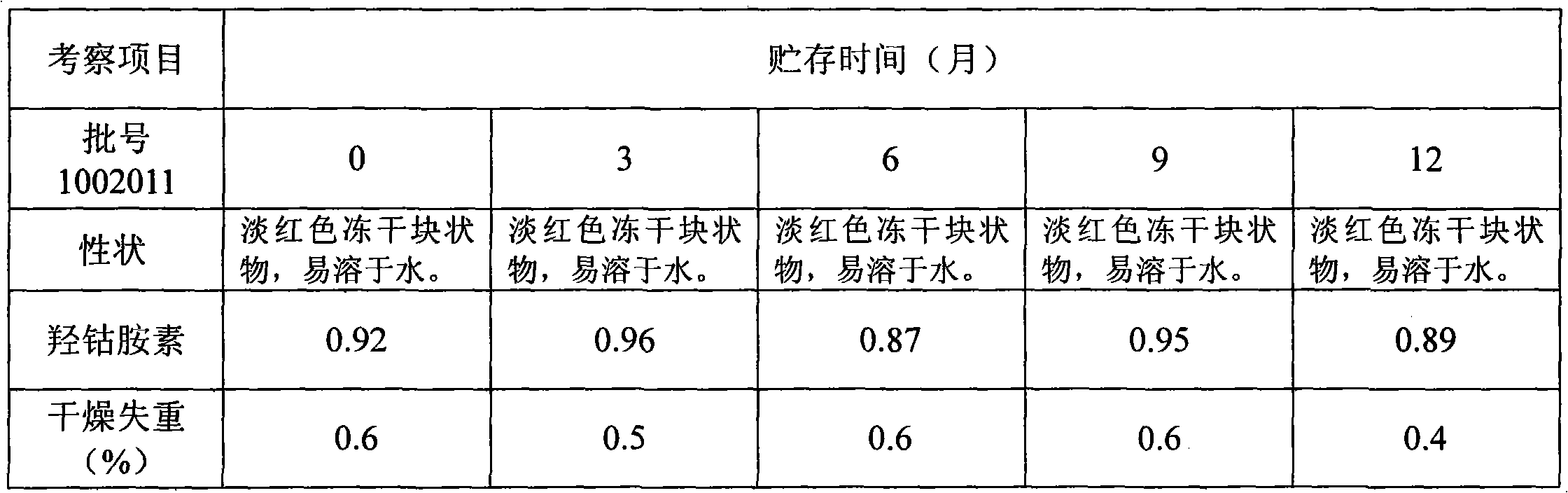
[0069] c. Measure the pH value and content of the solution obtained in "b". After passing the test, it is divided into 1000 vials, freeze-dried, and capped to obtain the combined injection freeze-dried preparation of adenosylcobalamin and adenosine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com