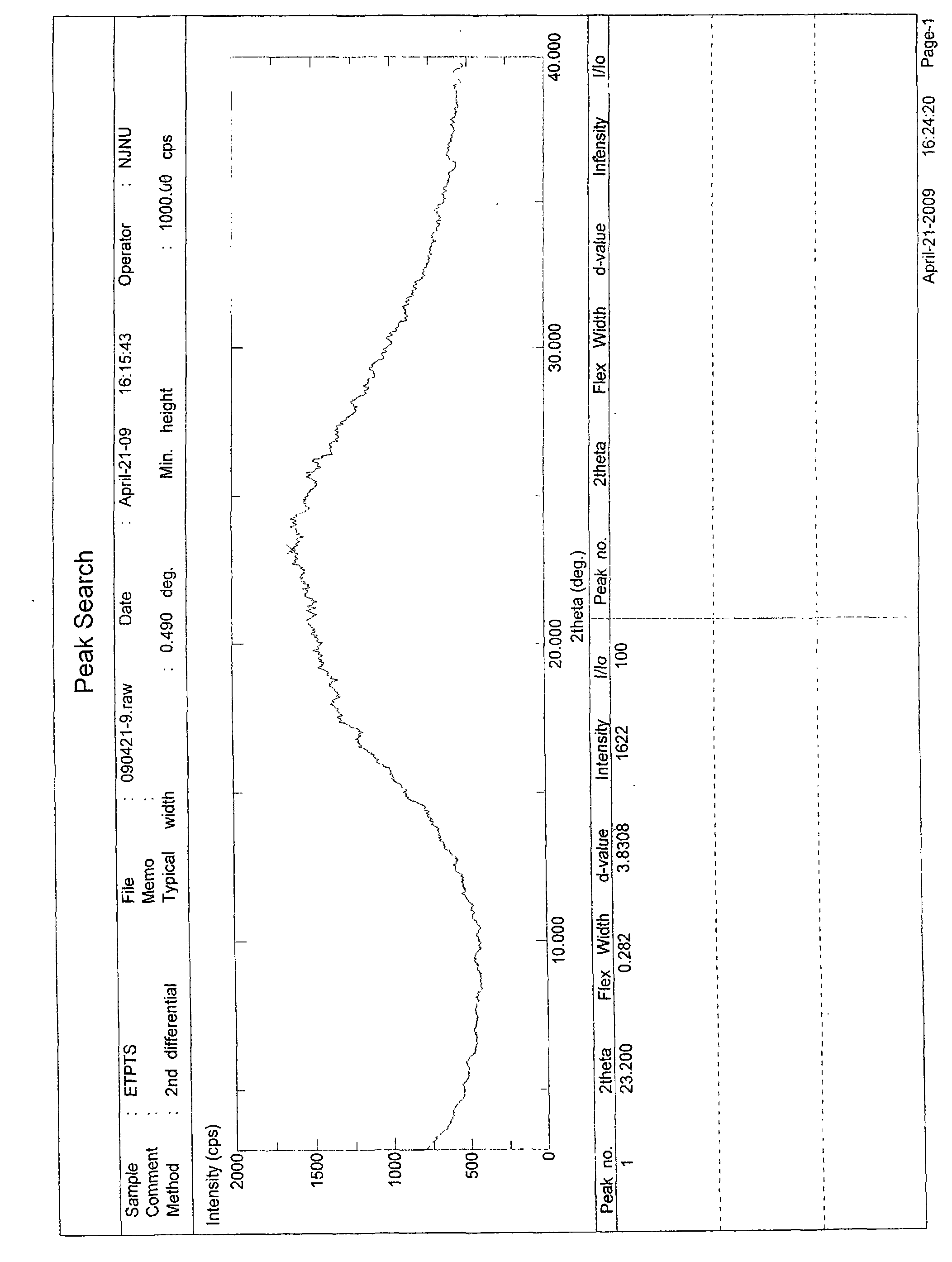
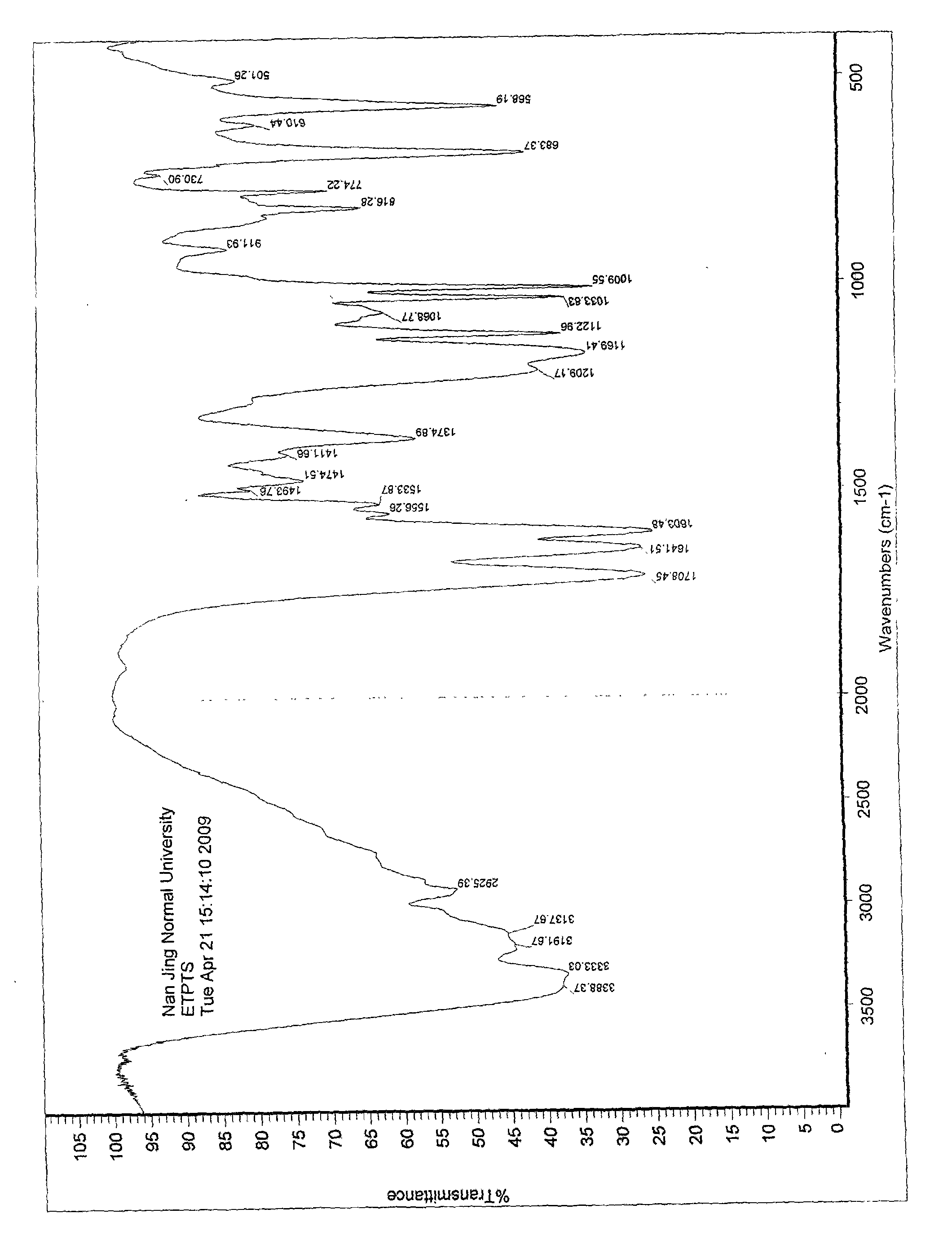
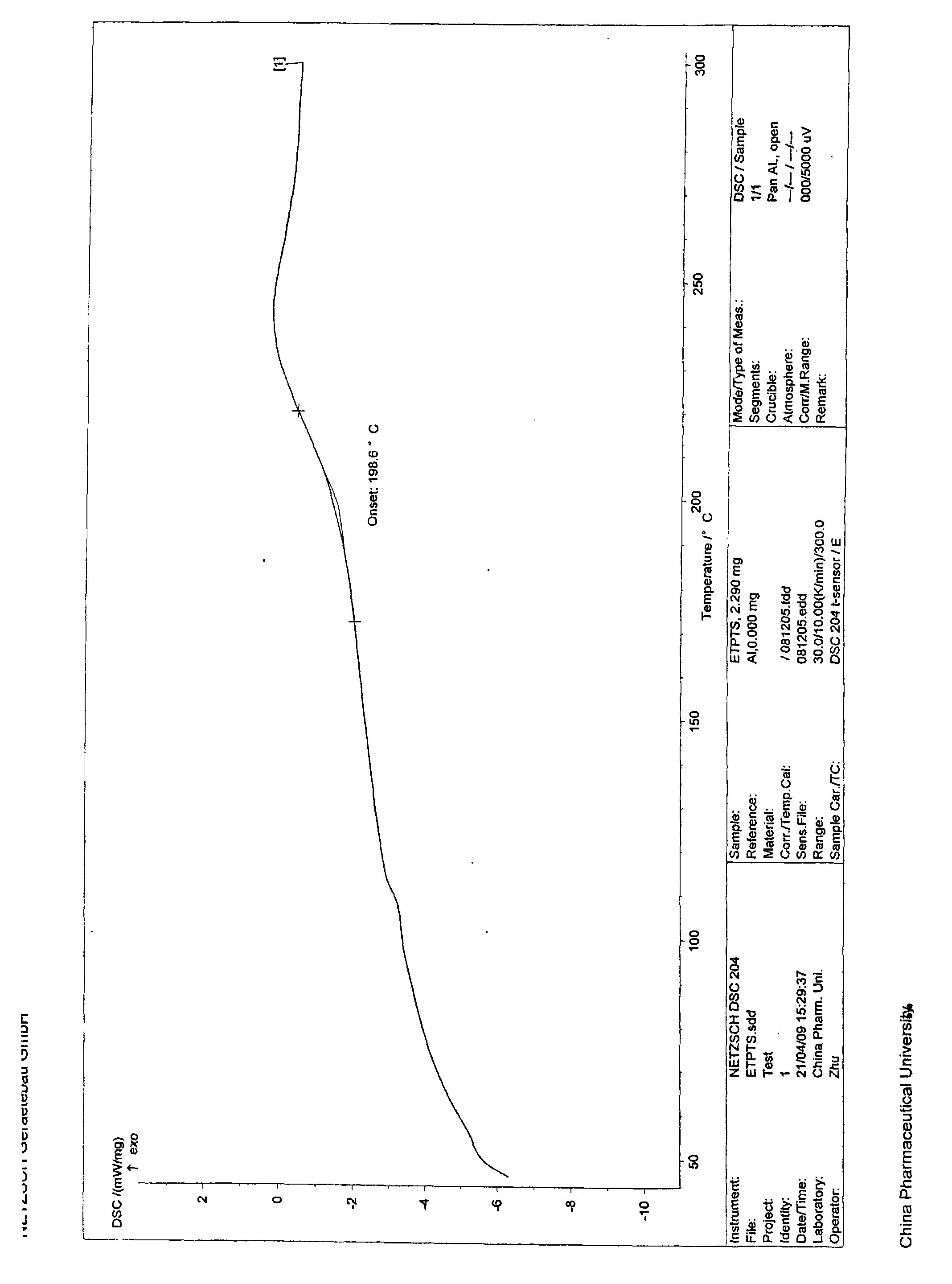
Amorphous entecavir p-toluenesulfonate salt and preparation method and medicament application thereof
A technology of p-toluenesulfonate and entecavir, applied in the field of medicine, can solve problems such as differences in bioavailability, and achieve the effects of good stability, improved production quality and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Put 2.95g of entecavir monohydrate in a reaction flask, add 30.0ml of methanol, stir, heat the reaction solution to 40°C, add a solution made of 2.0g of p-toluenesulfonic acid and 5.0ml of methanol in batches, after the addition, 65 React at ℃ for 3 hours, concentrate under reduced pressure to 12.5ml, cool to 0℃, add 130.0ml ethyl acetate, a viscous solid precipitates, pour off the solvent, wash with 4.0ml ethyl acetate, and dry the residue in vacuum to obtain a white foam Solid para-amorphous entecavir p-toluenesulfonate 4.45g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com