New method for combining ethyl sulfonamide
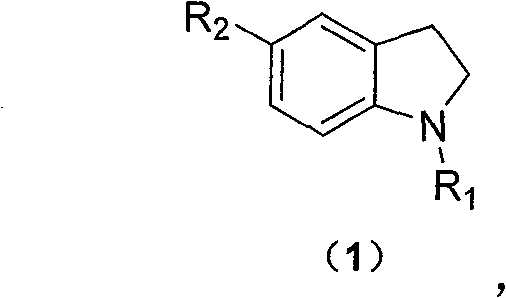
A technology of ethanesulfonamide and tert-butoxycarbonylmethanesulfonamide, applied in the synthesis of N-methyl-1H-indole-5-ethanesulfonamide, synthesis of ethanesulfonamide, synthesis of N-methyl-1H-indole - The field of 5-ethanesulfonamide compounds can solve the problems of short steps, cheap and easy-to-obtain raw materials, etc., and achieve the effect of short steps, cheap and easy-to-get raw materials, and less side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
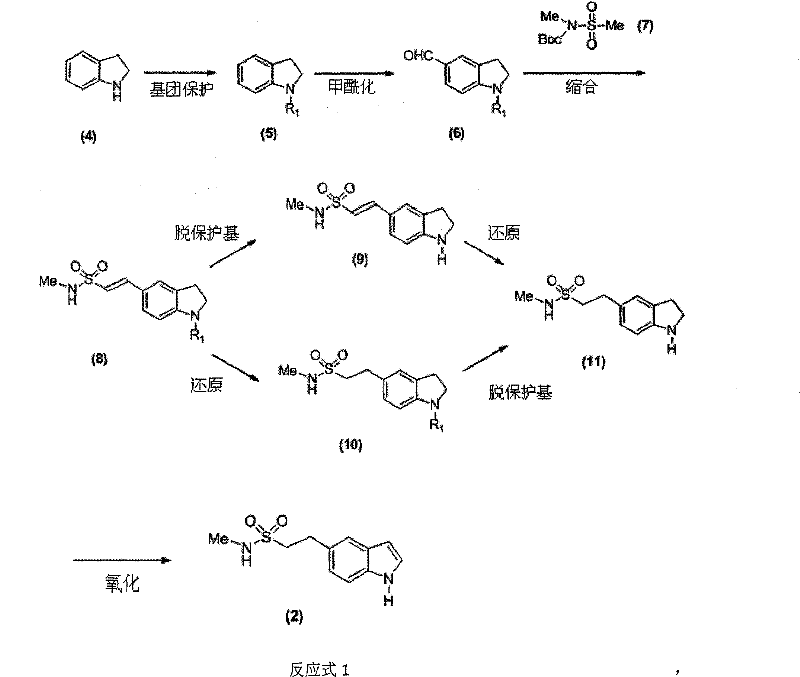
[0048] 1. Synthesis of N-benzyl indoline (5)
[0049] In a 250ml four-necked flask equipped with a dropping funnel and a thermometer, add indoline (4) (50g, 0.42mol), K 2 CO 3 (70g, 0.51mol) and DMF (120ml), stirred and warmed to 100°C. Benzyl bromide (75.42 g, 0.44 mol) was slowly added dropwise over 1 hour. After the dropwise addition was completed, the reaction was incubated for 3 hours, and TLC showed that the reaction was complete. The reaction solution was cooled to room temperature, poured into 1000ml of water, stirred for 0.5 hours, and then separated into layers, and the black oily substance in the lower layer was separated. After stirring and standing, solids would precipitate out, and recrystallized from ethyl acetate to obtain 80.12 g of compound (5), with a yield of 91.3%.
[0050] 2. Synthesis of N-benzyl-5-formaldehyde indoline (6)
[0051] In a 100ml four-necked flask equipped with a dropping funnel and a thermometer, add DMF (20ml), cool the system to bel...
Embodiment 2
[0070] Except for changing the synthesis conditions of compound (11), other steps were the same as in Example 1.
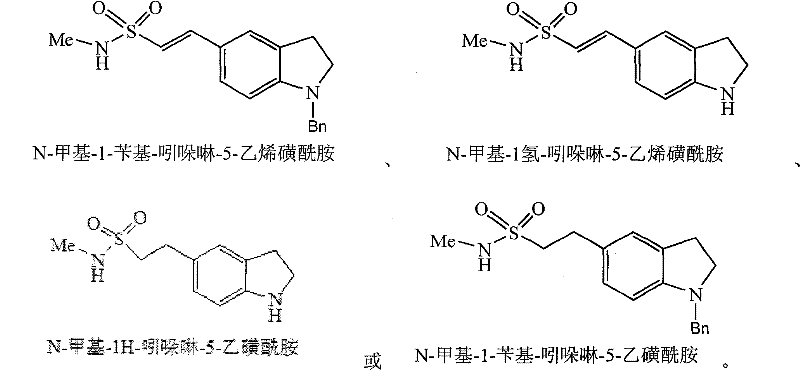
[0071] 1. Synthesis of N-methyl-1H-indoline-5-ethanesulfonamide (11)
[0072] Add compound (9) N-methyl-1H-indoline-5-ethylene sulfonamide (10.0g, 0.042mol) and ethanol 300ml in the hydrogenation kettle, add the Pd / C catalyst that the weight percent of Pd is 10% (5.0g), adjust the hydrogen pressure to 0.5MP, stir and hydrogenate at room temperature for 10 hours. The reaction ends when the hydrogen uptake stops. The Pd / C catalyst was recovered by filtration. The organic solvent in the filtrate was evaporated under reduced pressure, and 8.2 g of compound (11) N-methyl-1H-indoline-5-ethanesulfonamide was obtained by recrystallization, with a yield of 81.3%.
[0073] 2. Synthesis of N-methyl-1H-indoline-5-ethanesulfonamide (11)
[0074] Add compound (8) N-methyl-1-benzyl-indoline-5-ethylenesulfonamide (38.2g, 0.10mol) and THF 500ml in the hydrogenation kettle, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com