Application of 2-phenylphenol and derivatives thereof to medicine for treating epilepsia
A technology of biphenol and derivatives, applied in the field of medicine, to achieve the effects of strong affinity, excitotoxicity protection, and brain protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
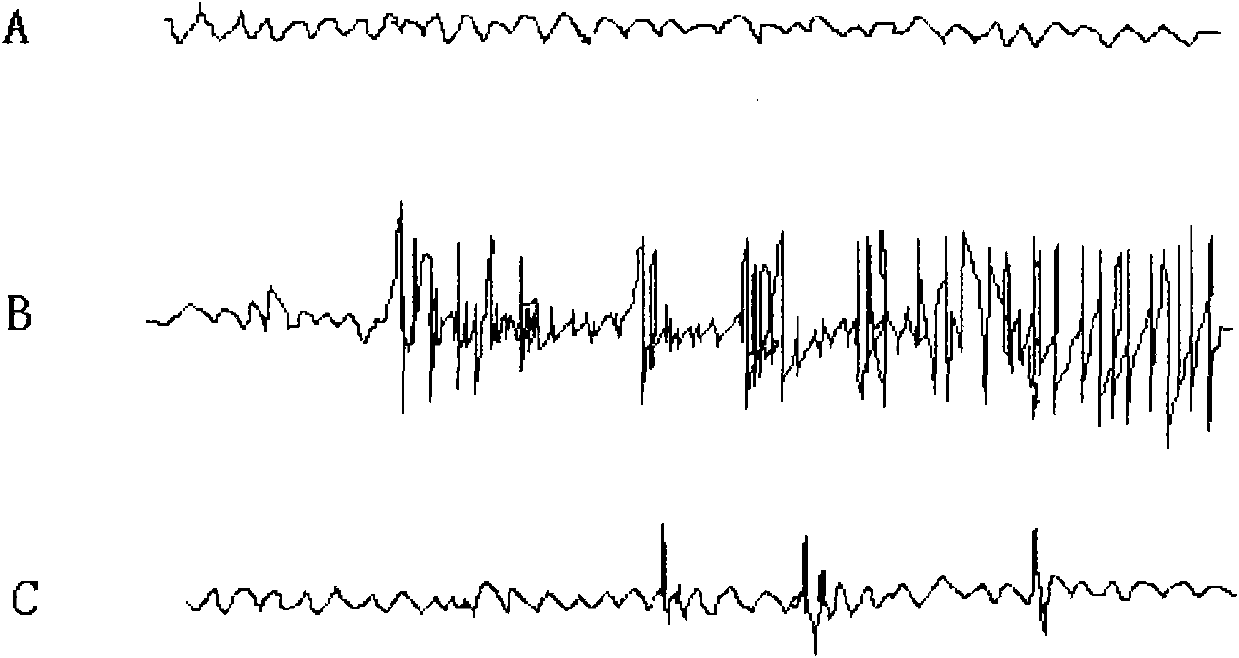
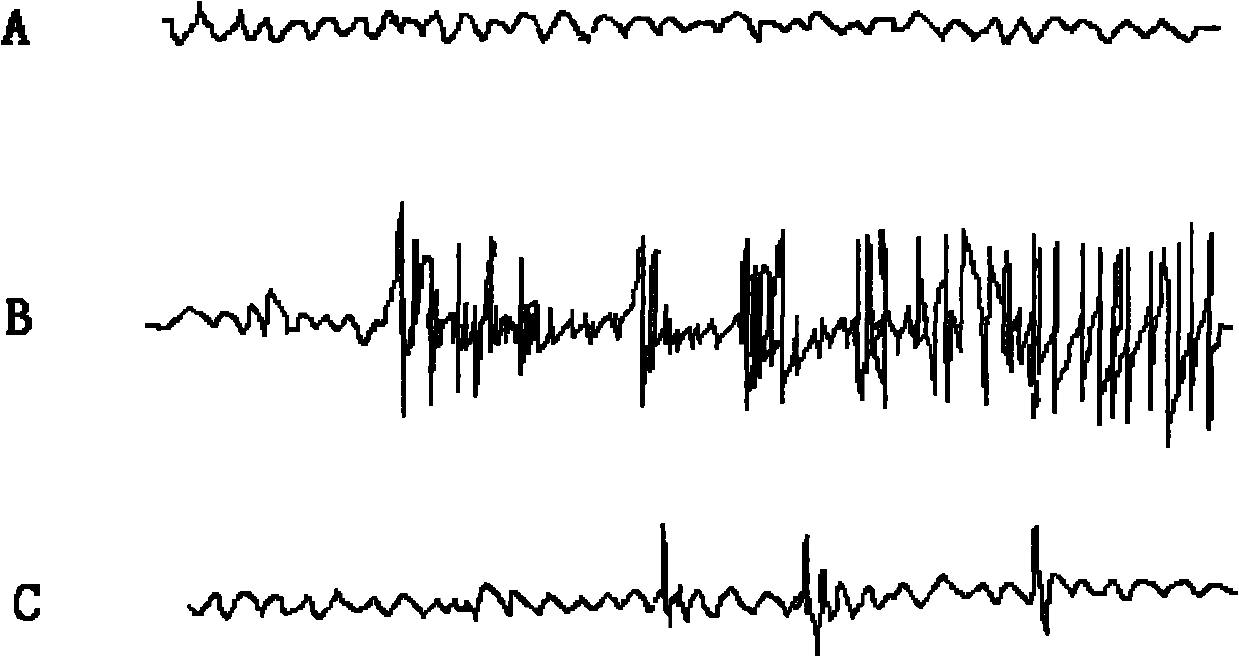
[0027] The effect of 2,2',6,6'-tetraisopropyl-4,4'-biphenol on the cortical EEG of pentylenetetrazol epilepsy model rats during the onset period. Material: male SD rats, body weight 250~ 300g, purchased from the Experimental Animal Center of the Fourth Military Medical University. Pentylenetetrazol (Sigma, USA).
[0028] Main instruments: multi-channel physiological recorder, special electrode, dental bench drill, general electroencephalogram.
[0029] Method:
[0030] (1) Preparation of pentylenetetrazol epilepsy model
[0031] 35mg / kg pentylenetetrazol was injected intraperitoneally to induce seizures.
[0032] (2) Pentylenetetrazol epilepsy model rat cortical EEG recording during seizure
[0033] First, the rats are anesthetized with isopentobarbital. Cut the scalp to expose the skull, drill a small hole on the left and right with a dental electric drill 3mm next to the junction of the coronal suture and the sagittal suture, implant electrodes, fix the electrodes and suture the sca...
Embodiment 2
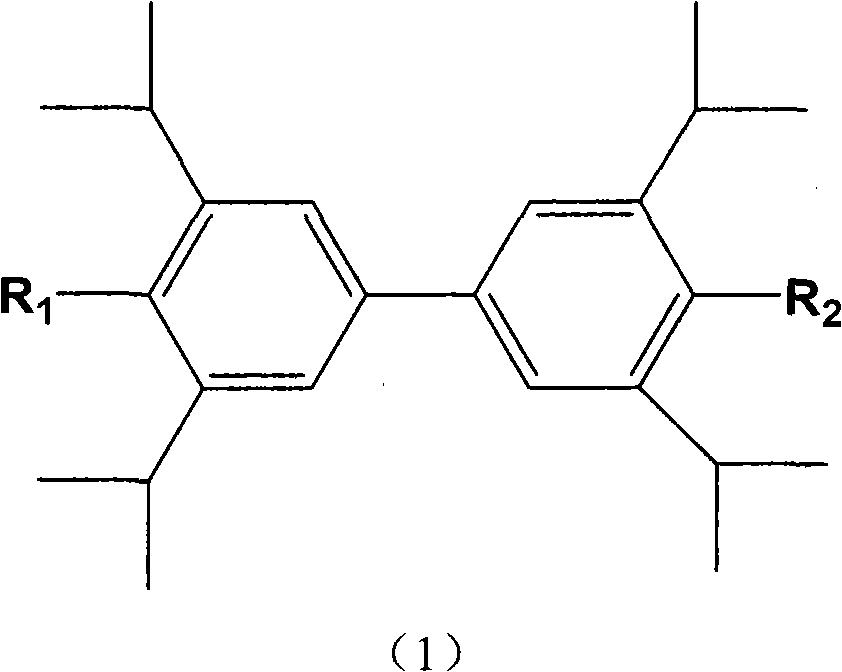
[0042] The other diphenols or derivatives thereof according to the present invention also have the same therapeutic effect as the 2,2',6,6'-tetraisopropyl-4,4'-diphenols described in Example 1.
Embodiment 3
[0043] Example 3: Pharmaceutical composition
[0044] 2,2',6,6'-Tetraisopropyl-4,4'-Biphenol is passed through a 100 mesh sieve for later use; 2,2',6,6'-Tetraisopropyl-4,4'- Diphenol 80.0g, microcrystalline cellulose 45.0g, croscarmellose sodium 5.0g, mixed uniformly, made of soft material with aqueous solution as wetting agent, granulated by 30 mesh screen, ventilated and dried at 55℃, Granules, ready for use; add 0.5 g of micro-powder silica gel, 1.0 g of magnesium stearate and mix well, and press to obtain about 1000 round tablets to obtain compressed tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com