Method for the production of a nano-scale silicon dioxide
A technology of silicon dioxide and colloidal silicon dioxide, which is applied in the fields of nanotechnology, nanotechnology, nanotechnology for materials and surface science, etc. It can solve the problem that large-scale production is difficult to control, the cost is high, and it cannot be clearly produced. Single-state narrow particle size distribution of SiO2 fillers, etc., to achieve the effect of good thermal conductivity and similar properties, easy processing, stable storage and stable dispersion system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
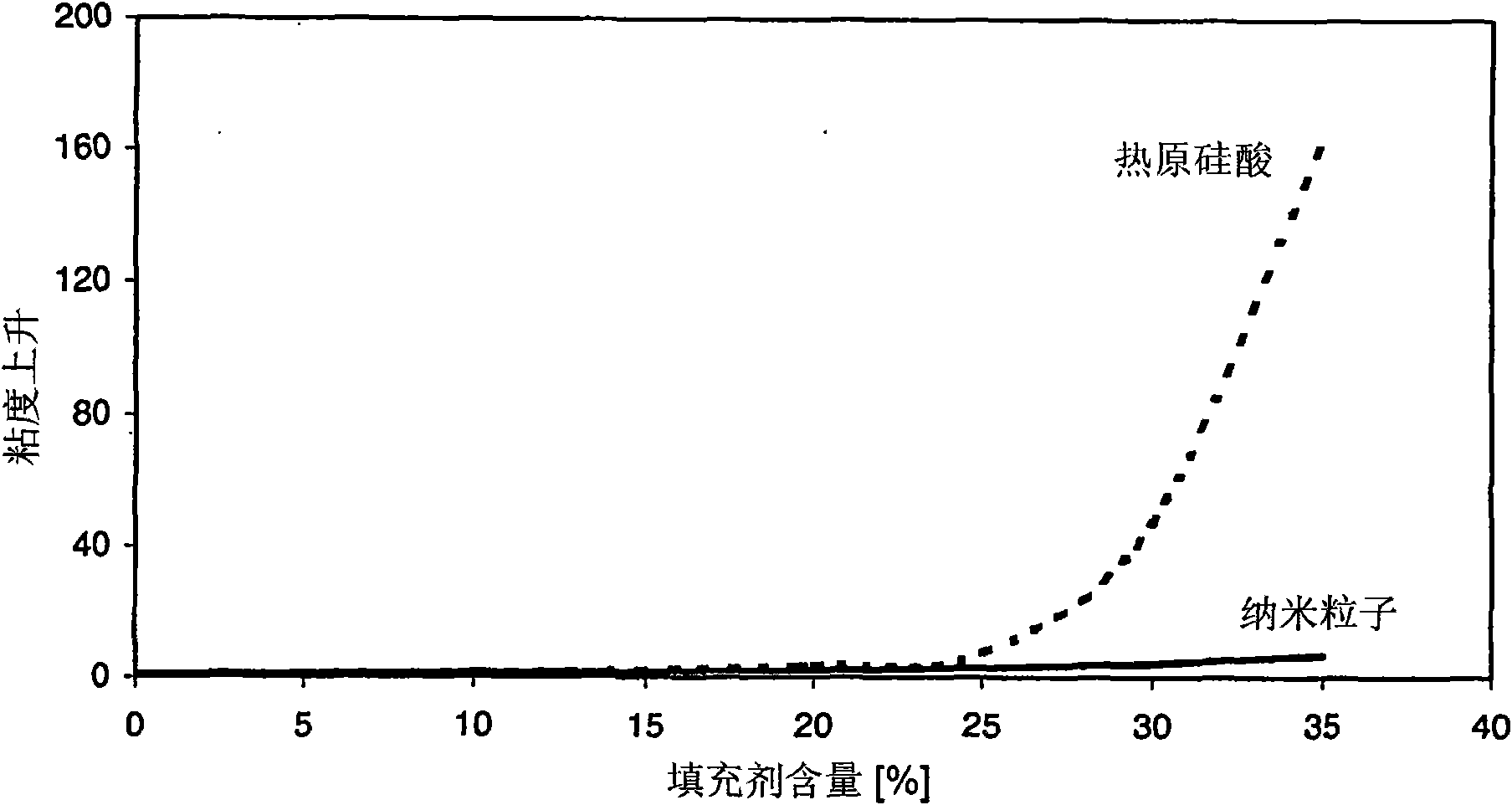
Method used
Image
Examples
Embodiment 1
[0085] Preparation of Dispersion System of Silica in Toluene
[0086] Add 63g trimethylchlorosilane in 1260g THF in the three-neck flask, drop 1050g silica sol (Levasil 200 / 40%, BET=200m 2 / g, 40% SiO 2 , Na + removed with ion exchangers).
[0087]Within one hour two phases formed which were separated with a separatory funnel. The lower phase accounts for more than 99% of the solids, and the upper phase accounts for most of the water. The lower phase was diluted with 140 g of THF, and 63 g of trimethylchlorosilane was added with stirring. After stirring for one hour it was transferred to a separatory funnel.
[0088] After one hour two more phases formed which were separated and drained. The upper phase consists mainly of water and THF.
[0089] The lower phase was transferred into a three-necked flask and diluted with 400 g of toluene. A mixture of THF, water and toluene is then distilled off with further addition of toluene. Toluene was added in such a way as not to...
Embodiment 2
[0092] Preparation of Dispersion System of Silica in THF
[0093] The preparation method is the same as in Example 1, except that the step of replacing THF with toluene by distilling off THF is omitted. Sols were obtained with a solids content between 45% and 55% by weight. The THF sol was heated under reflux for 6 hours to neutralize it, and a basic ion exchanger (Amberjet 4400 OH from Rohm & Haas Company) was used to guide the reflux solvent.
Embodiment 3
[0095] Preparation of Dispersions of Vinyl-functionalized Silica in Toluene
[0096] The preparation method is as in Example 1. Only dimethylvinylchlorosilane (DMVSC1) replaces trimethylchlorosilane (TMSC1) completely or partially in the first and / or second silanization step.
[0097] A total of nine experiments were carried out using different ratios of the two silanes in the first and second silanizations, see Table 1 for details.
[0098] Table 1
[0099]
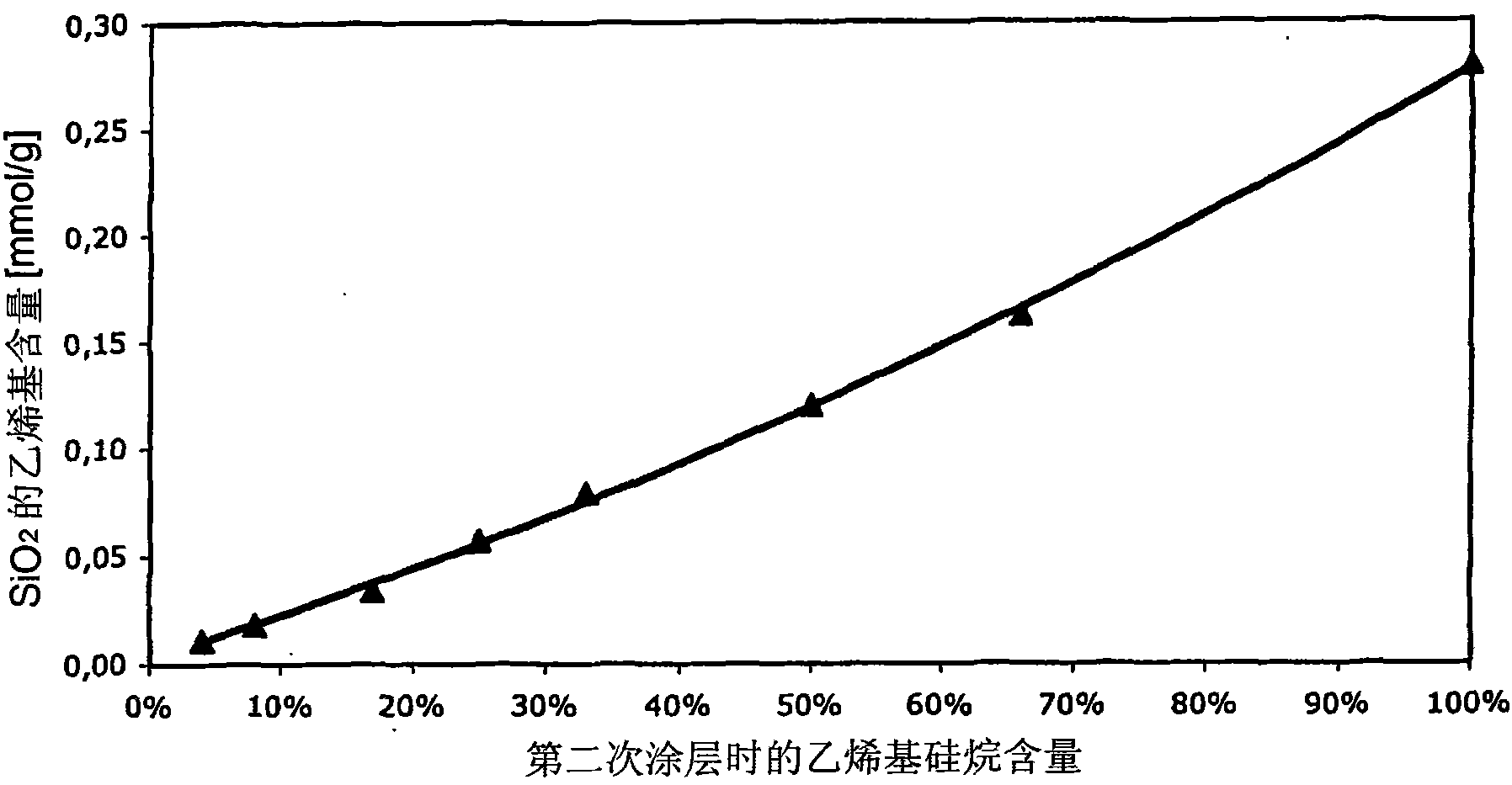
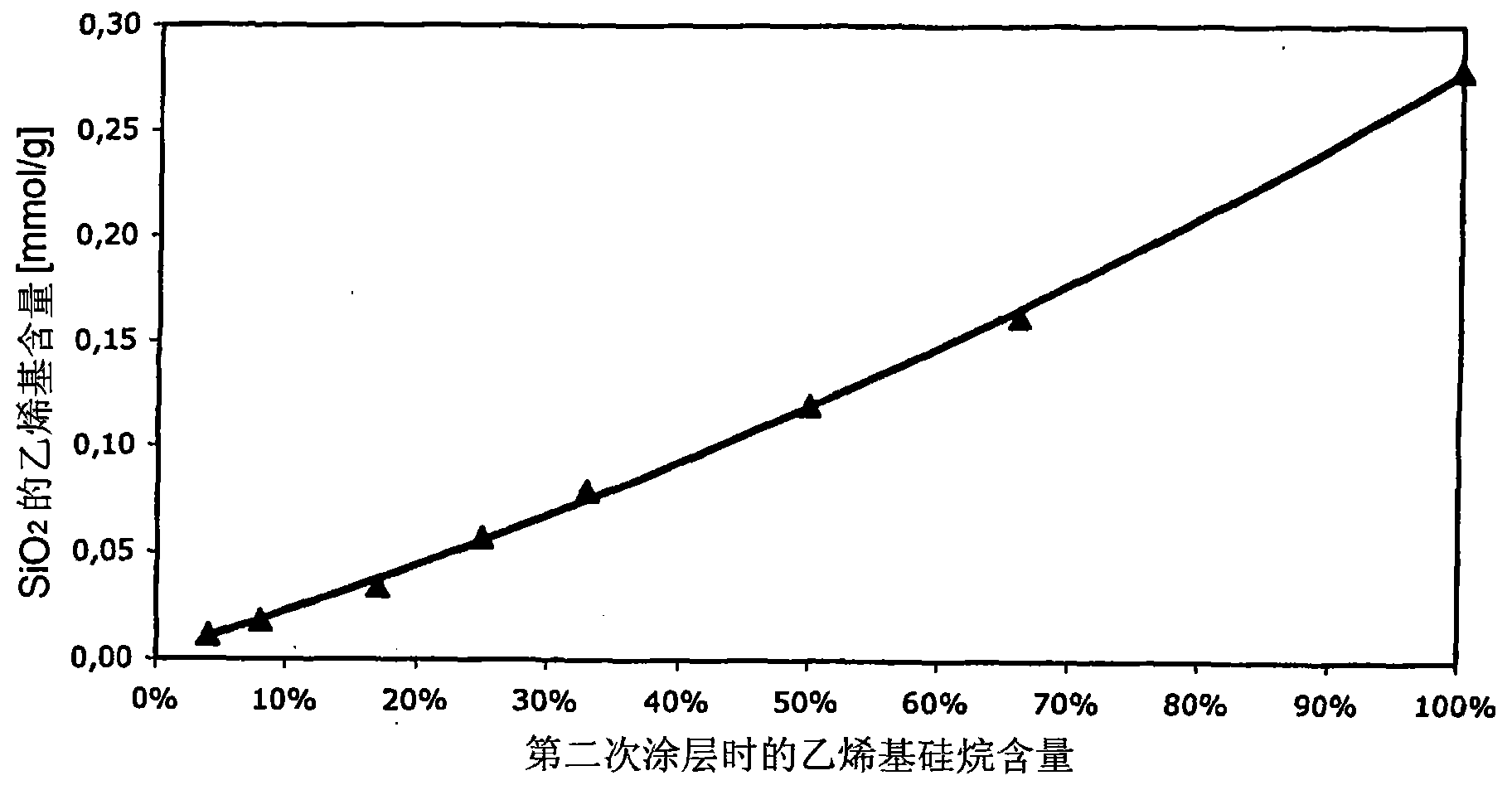
[0100] As can be seen from the table above, the use of dimethylvinylchlorosilane in the second silanization increases the vinyl content of the surface relative to the use of an equivalent amount of trimethylvinylchlorosilane in the first silanization. Degree of group functionalization (Examples 3.1 and 3.2).
[0101] figure 1 Shown is SiO 2 Graph of the degree of functionalization of the surface versus the amount of dimethylvinylchlorosilane used in the second silylation step.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com