Preparation method of 2,3-anhydride naphthalene derivative
A compound, -NO2 technology, applied in 2 fields, can solve the problems of difficult purification, long synthesis route, poor reaction selectivity, etc., and achieve the effect of simple synthesis route
Inactive Publication Date: 2010-08-25
EAST CHINA UNIV OF SCI & TECH
View PDF4 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
This method has defects such as long synthetic route, poor reaction selectivity (such as a large amount of isomers will be produced in the second step reaction) and difficult purification.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Login to View More
Abstract
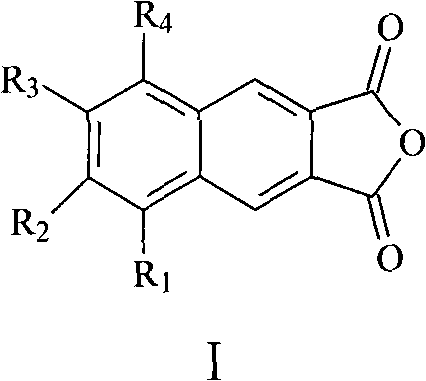
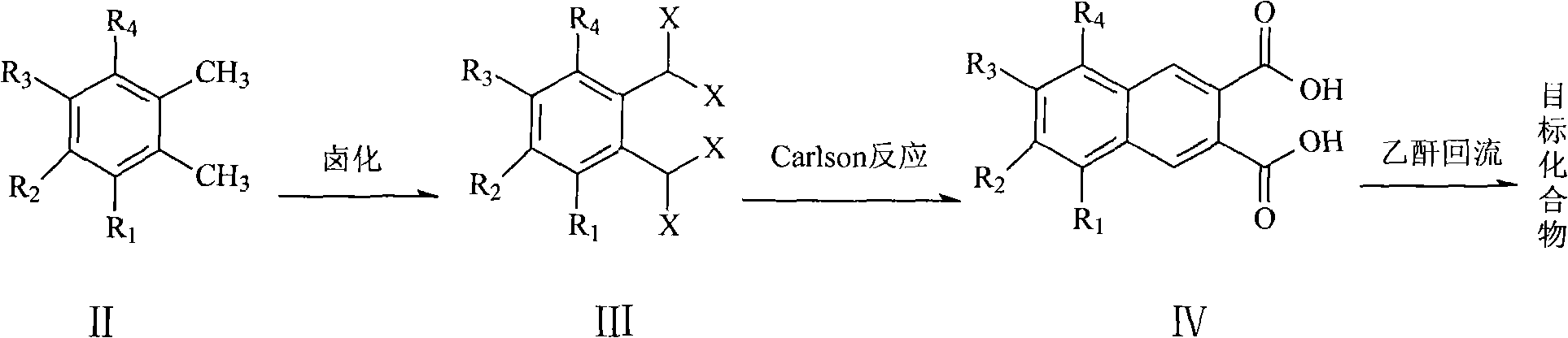
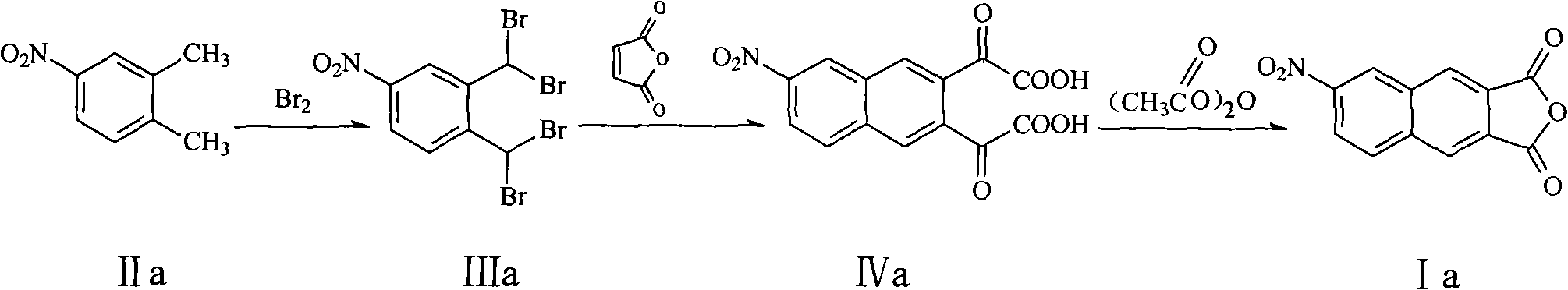
The invention relates to a preparation method of a 2,3-anhydride naphthalene derivative, which comprises the main steps of: sequentially carrying out a halogenation reaction, a Carlson reaction and a condensation reaction (refluxed with acetic anhydride) by using an ortho-xylene derivative as an initial raw material for obtaining a target compound. The preparation method overcomes the defects of complicated step and low total yield in a traditional method for preparing the 2,3-anhydride naphthalene derivative, and has the advantages of simple and direct synthesizing route, mild reaction condition of each step, high yield of the target product and the like.
Description
technical field The invention relates to a preparation method of 2,3-naphthalene anhydride derivatives. Background technique Naphthalimides are an important class of heterocyclic compounds with wide applications. For example, 2,3-naphthoimide and 1,8-naphthoimide are used as fluorescent compounds in the biological field, such as probes for monitoring the binding of peptides to histocompatible proteins, selective opioid peptides, environmentally sensitive Fluorescent probes for peptide-peptide interaction, etc. (InVest. New Drugs 1992, 10, 177-181; Nucleic Acids Res. 1979, 7, 217-230; Cancer Res. 1995, 55, 1176-1180; Cancer Res. 1994, 54, 2199-2206; J. Natl. Cancer Inst. 1994, 86, 1462-1465). 2,3-naphthalene anhydride derivatives are an important intermediate for the preparation of 2,3-naphthoimide or its derivatives. Pub.No.: US2006 / 0234206A1 (publication date 2006-10-19) discloses a preparation method of 2,3-naphthalene anhydride derivatives, the synthetic route of whic...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07D307/92
Inventor 钱旭红巴布徐玉芳
Owner EAST CHINA UNIV OF SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com