Synthesis method of antineoplastic medicine capecitabine
An anti-tumor drug, capecitabine technology, applied in the field of medicinal chemistry, can solve the problems of expensive starting materials, increased production costs, etc., and achieves easy industrial production and popularization and application, short operation time and high reaction yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
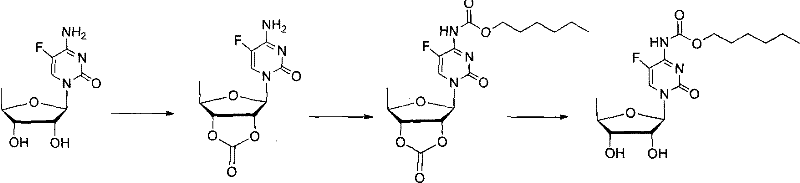
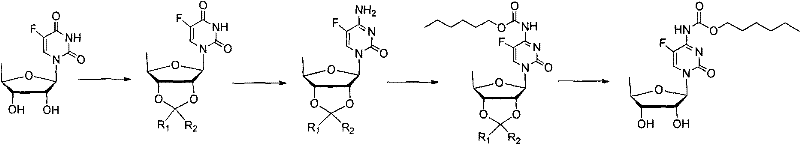
Examples
Embodiment 1
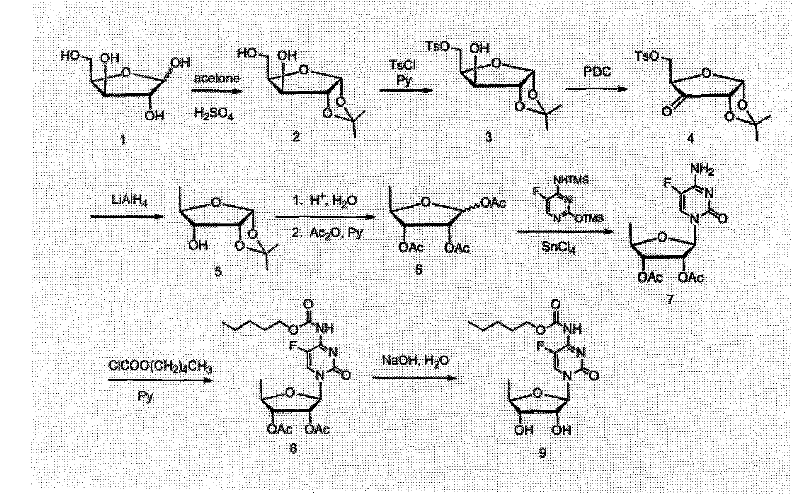
[0027] 1. Preparation of Compound 2
[0028] Add xylose (100g, 0.67mol) into 1L of acetone, add concentrated sulfuric acid (30ml, 0.55mol) dropwise, stir at room temperature for 8 hours, adjust the pH to 3 with saturated sodium bicarbonate solution, stir for 2 hours, Adjust the pH to neutral, filter and concentrate to obtain compound 2 (116.5 g), with a yield of 92.0%.
[0029] 2. Preparation of Compound 3
[0030] Compound 2 (30g, 0.16mol) was dissolved in 200ml of pyridine, p-toluenesulfonyl chloride (31.9g, 0.17mol) was added in portions within 2 hours, then stirred for 1 hour, 150ml of saturated sodium bicarbonate solution and 300ml of dichloromethane, stirred for 30 minutes, separated the organic phase, washed the organic phase with saturated sodium bicarbonate solution and saturated brine, dried over anhydrous magnesium sulfate, filtered and concentrated to obtain a light yellow solid, which was crystallized by adding ether to obtain compound 4. White crystals (39.1 g)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com