Conjugate of hapten and amino-containing carrier and preparation method and usage thereof
A hapten and amino group-containing technology, which is applied in the direction of carrier-binding antigen/hapten components, anti-inflammatory agents, biological testing, etc., can solve the problems of limited targeted immunity and failure to prevent and treat chronic infections, and achieve enhanced specific immunity, Improving the effect of the local immune microenvironment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
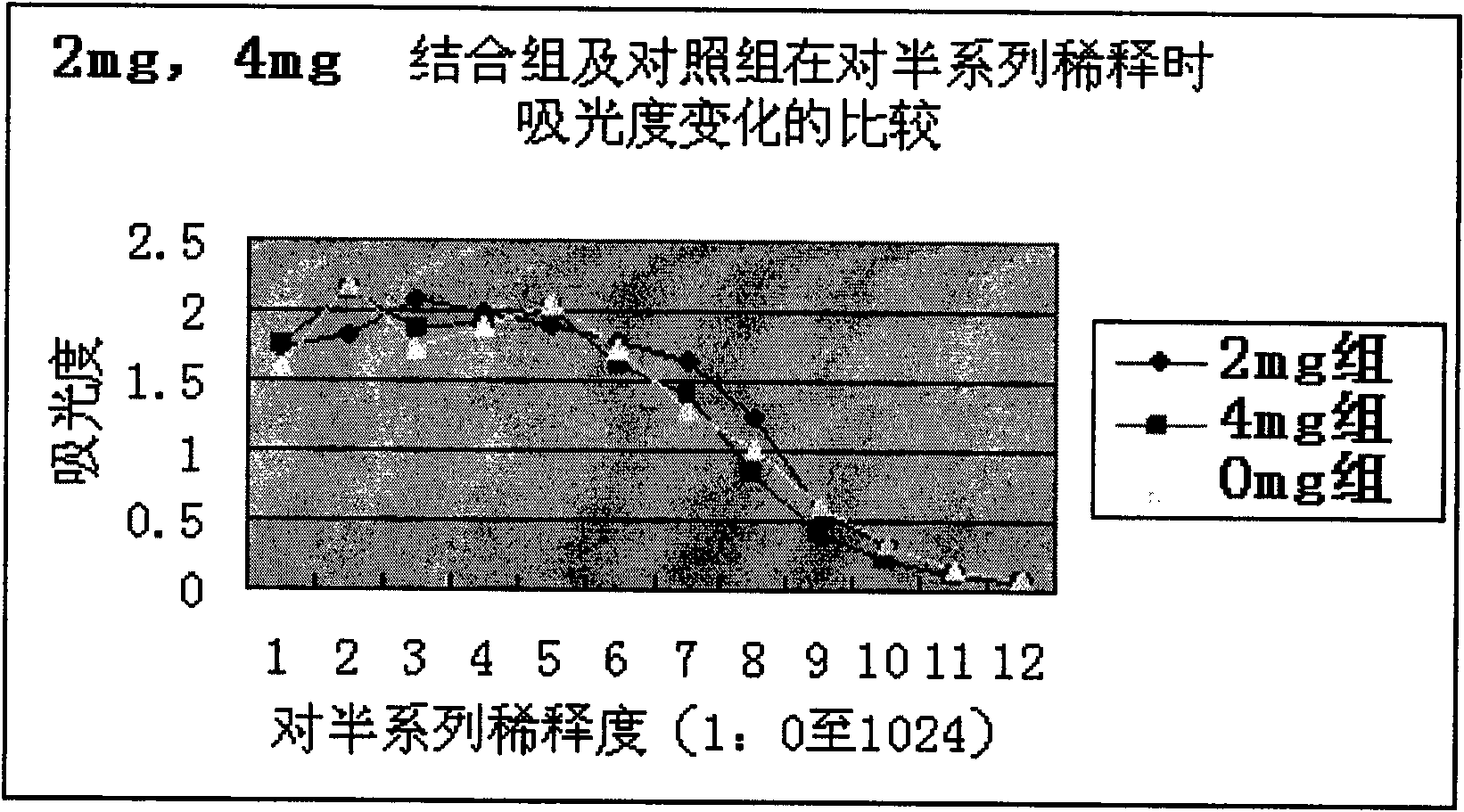
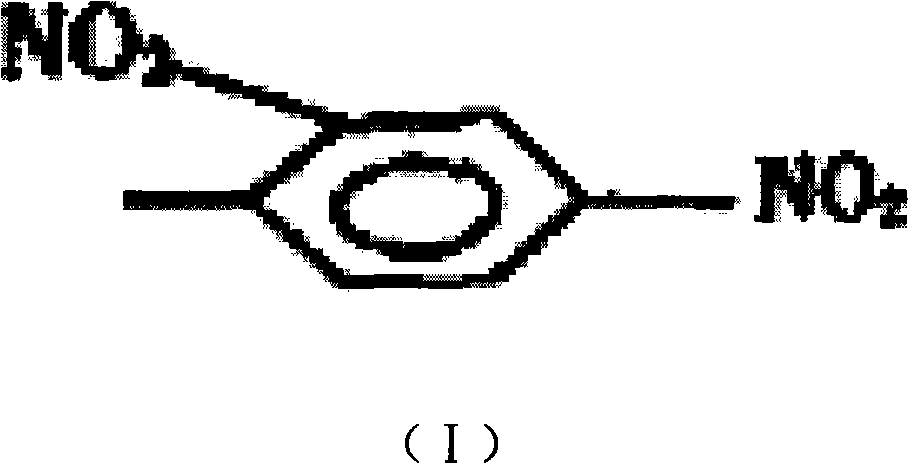
[0039]Using the hapten dinitrofluorobenzene and anti-hepatitis B surface antigen monoclonal antibody as reaction materials, under weakly alkaline conditions, through Sanger's reaction, the fluorine falls off the hydrogen on the amino group in the monoclonal antibody, and the dinitrobenzene Partially replace and covalently bind to the amino group to obtain the target product dinitrobenzene covalently bound to the hepatitis B surface antigen monoclonal antibody, one of the target products, the binding rate: 1-200 dinitrobenzene, the binding amount is not Affect the specific recognition function of monoclonal antibody.
[0040] Dinitrochlorobenzene, dinitrobromobenzene, or dinitroiodobenzene may also be used as the above-mentioned hapten.
[0041] After immunoassay (such as ELISA), the results show that the signal of the immunoreaction assay is amplified up to 200 times, which significantly improves the sensitivity of the immunoassay.
[0042] Mix the aqueous solution of HBIG (1...
Embodiment 2
[0045] According to the preparation method of Example 1, a tumor or infection targeting agent covalently bound by dinitrobenzene is used to prepare a hapten-induced immunotargeted therapy tumor drug, or / and directly use dinitrofluorobenzene as the original drug It has anticancer effect. The results showed that the binding ratio was the same as that in Example 1; the tumor inhibition rate of subcutaneously transplanted tumors in mice injected with DNFB was good.
[0046] The tumor or infection targeting preparation can be selected from anti-HER-2 monoclonal antibody Herceptin or hepatitis B monoclonal antibody.
Embodiment 3
[0048] According to the preparation method of Example 1, the immune antigen or epitope of the tumor or chronic infection pathogen covalently bonded with dinitrobenzene is used to prepare the prevention and treatment drug induced by hapten or to strengthen specific immunity. For example, DNFB combined with hepatitis B surface antigen, DNFB combined with melanoma tumor regression antigen epitope, etc., can be used for specific prevention or as a tumor therapeutic vaccine, etc. The results show that the binding ratio is the same as that of Example 1. The drug for targeted treatment of tumor or chronic infection is characterized in that the compound with targeting effect is used as the guiding part for binding tumor, and is covalently combined with the hapten determinant part to carry out targeted treatment of tumor or chronic infection.
[0049] Table 1: 48-hour cell viability of SMMC-7721 liver cancer cell line in vitro (MTT method, mean value of six wells).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com