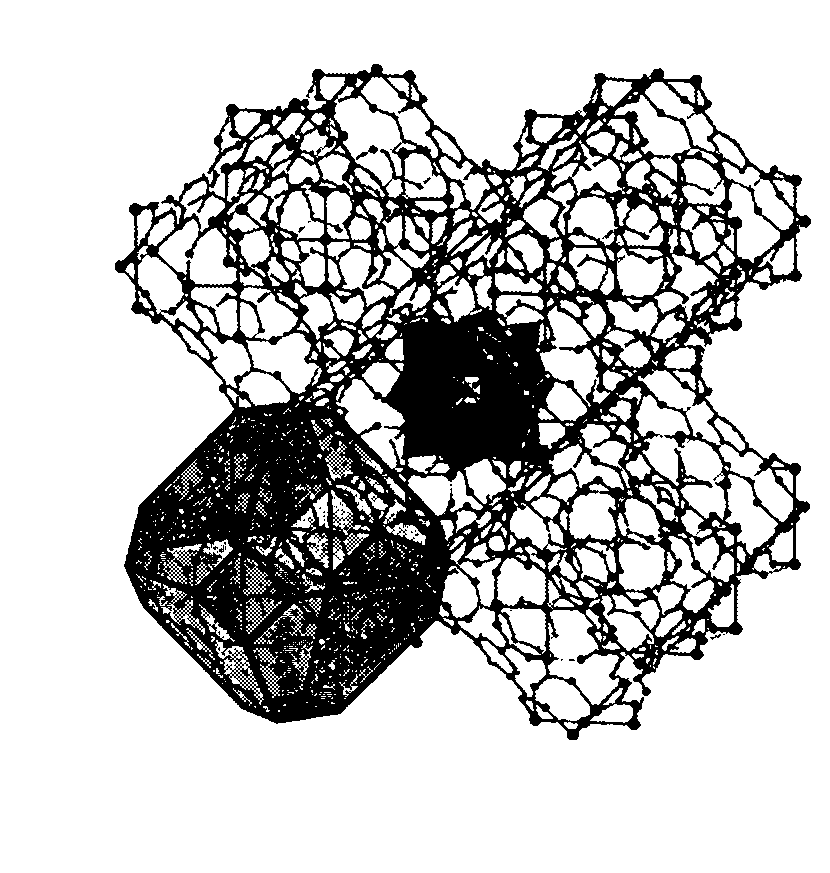
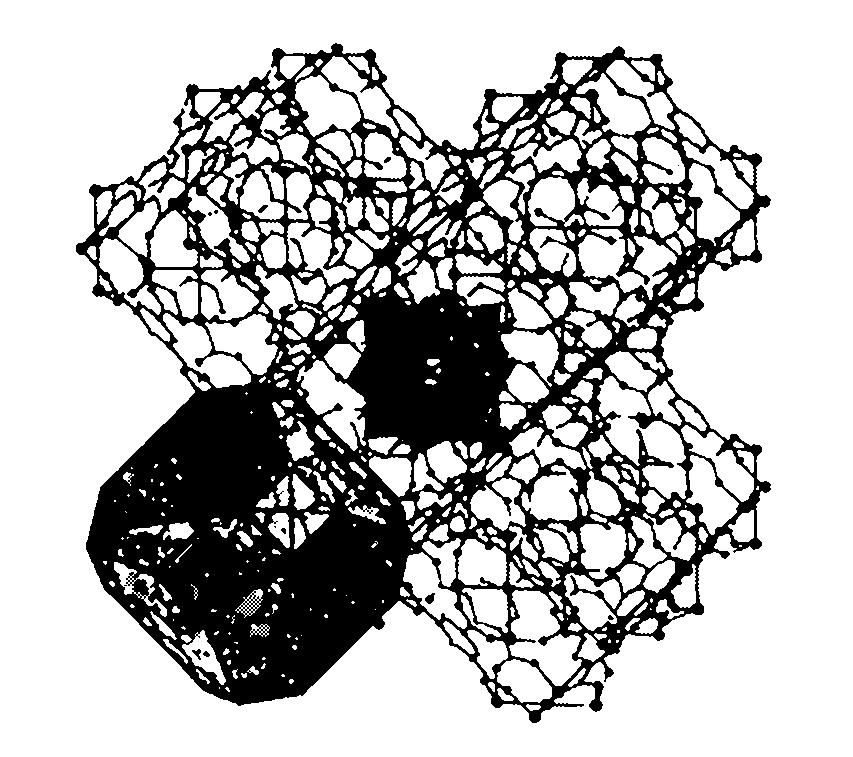
Supported polyoxometallate crystalline catalyst and preparation method thereof
A polyoxometalate and catalyst technology, applied in the field of chemical materials, can solve the problems of difficulty in catalyst separation and recovery, limit the catalytic performance of solid catalysts, and small specific surface area, and achieve obvious industrial application prospects, low cost, and simple preparation methods Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Example 1: H 3 [(Cu 4 Cl) 3 (BTC) 8 ] 2 [PW 12 o 40 ]·(C 4 h 12 N) 6 ·3H 2 Preparation of O crystalline catalyst
[0017] Na was prepared according to the literature method 3 PW 12 o 40 ·xH 2 O (Rocchiccioli-Deltcheff et al. Inorg. Chem. 1983, 22, 207-216). CuCl 2 2H 2 O, Na 3 PW 12 o 40 ·xH 2 O. Trimellitic acid and tetramethylammonium hydroxide were mixed in water at a material ratio of 9:1:10:20, and the stirring was continued at room temperature. The final pH value of the mixture was 4. The mixture was charged into a Teflon-lined autoclave and heated at 180°C for 50 hours to obtain olive green cubic crystals. After fully washing with deionization, it was collected by filtration to obtain pure compound H 3 [(Cu 4 Cl) 3 (BTC) 8 ] 2 [PW 12 o 40 ]·(C 4 h 12 N) 6 ·3H 2 O, the crystal structure belongs to the cubic crystal system, the space group Fm-3m, and the unit cell parameters are as follows: α=β=γ=90°,
Embodiment 2
[0018] Example 2: H 3 [(Cu 4 Cl) 3 (BTC) 8 ] 2 [PMo 12 o 40 ]·(C 4 h 12 N) 6 Preparation of Crystalline Catalysts
[0019] Na was prepared according to the literature method 2 HPMo 12 o 40 14H 2 O (Rocchiccioli-Deltcheff et al. Inorg. Chem. 1983, 22, 207-216). CuCl 2 2H 2 O, Na 2 HPMo 12 o 40 14H 2 O. Trimellitic acid and tetramethylammonium hydroxide were mixed in water according to the mass ratio of 10:1:8:16, and the stirring was continued at room temperature, and the final pH value of the mixture was 4.5. The mixture was charged into a Teflon-lined autoclave and heated at 180 °C for 72 hours to obtain green cubic crystals. After fully washing with deionization, it was collected by filtration to obtain pure compound H 3 [(Cu 4 Cl) 3 (BTC) 8 ] 2 [PMo 12 o 40 ]·(C 4 h 12 N) 6 , the crystal structure belongs to the cubic crystal system, the space group Fm-3m, and the unit cell parameters are as follows: α=β=γ=90°,
Embodiment 3
[0020] Example 3: H 3 [(Cu 4 Cl) 3 (BTC) 8 ] 2 [AsW 12 o 40 ]·(C 4 h 12 N) 6 ·3H 2 Preparation of O crystalline catalyst
[0021] Na was prepared according to the literature method 3 AsW 12 o 40 ·xH 2 O (Rocchiccioli-Deltcheff et al. Inorg. Chem. 1983, 22, 207-216). CuCl2 2H 2 O, Na 3 AsW 12 o 40 ·xH 2 O. Trimellitic acid and tetramethylammonium hydroxide were mixed in water according to the mass ratio of 8:1:10:20, and the stirring was continued at room temperature, and the final pH value of the mixture was 5. The mixture was charged into a Teflon-lined autoclave and heated at 195 °C for 50 hours to obtain blue cubic crystals. After fully washing with deionization, it was collected by filtration to obtain pure compound H 3 [(Cu 4 Cl) 3 (BTC) 8 ] 2 [AsW 12 o 40 ]·(C 4 h 12 N) 6 ·3H 2 O, the crystal structure belongs to the cubic crystal system, the space group Fm-3m, and the unit cell parameters are as follows: α=β=γ=90°,
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal stability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com