Method for preparing pentamethyl disiloxane
A technology of pentamethyldisiloxane and methyl, which is applied in the field of preparation of pentamethyldisiloxane, can solve the problems of low yield and high product cost, achieve high hydrolysis purity, good industrial value, and increase yield and the effect of purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
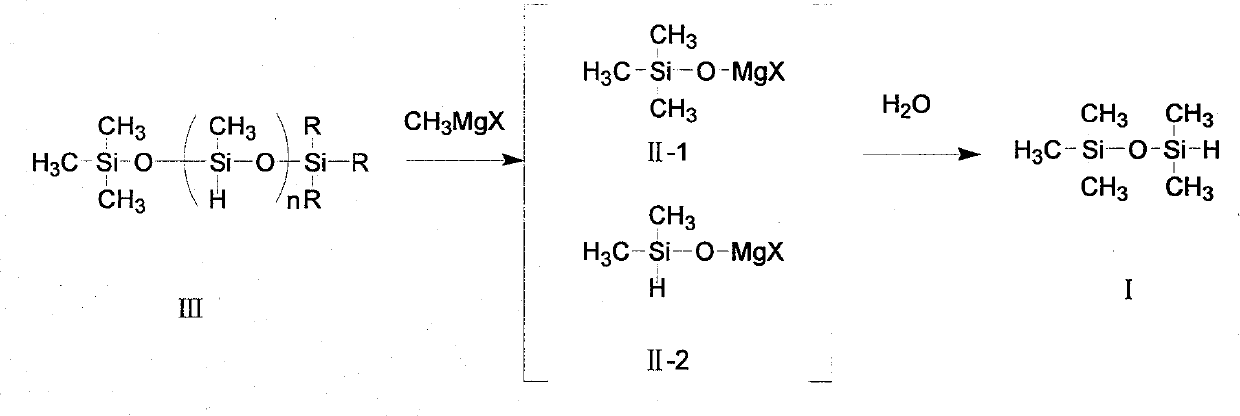
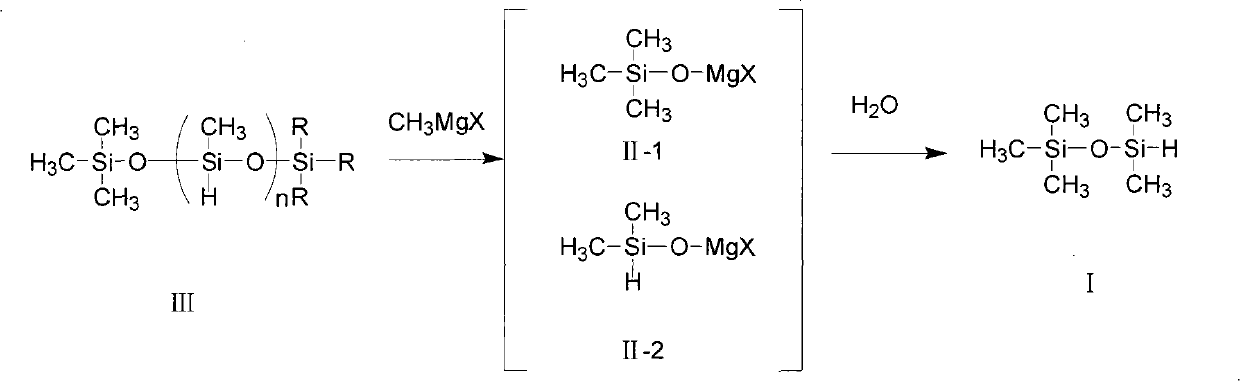
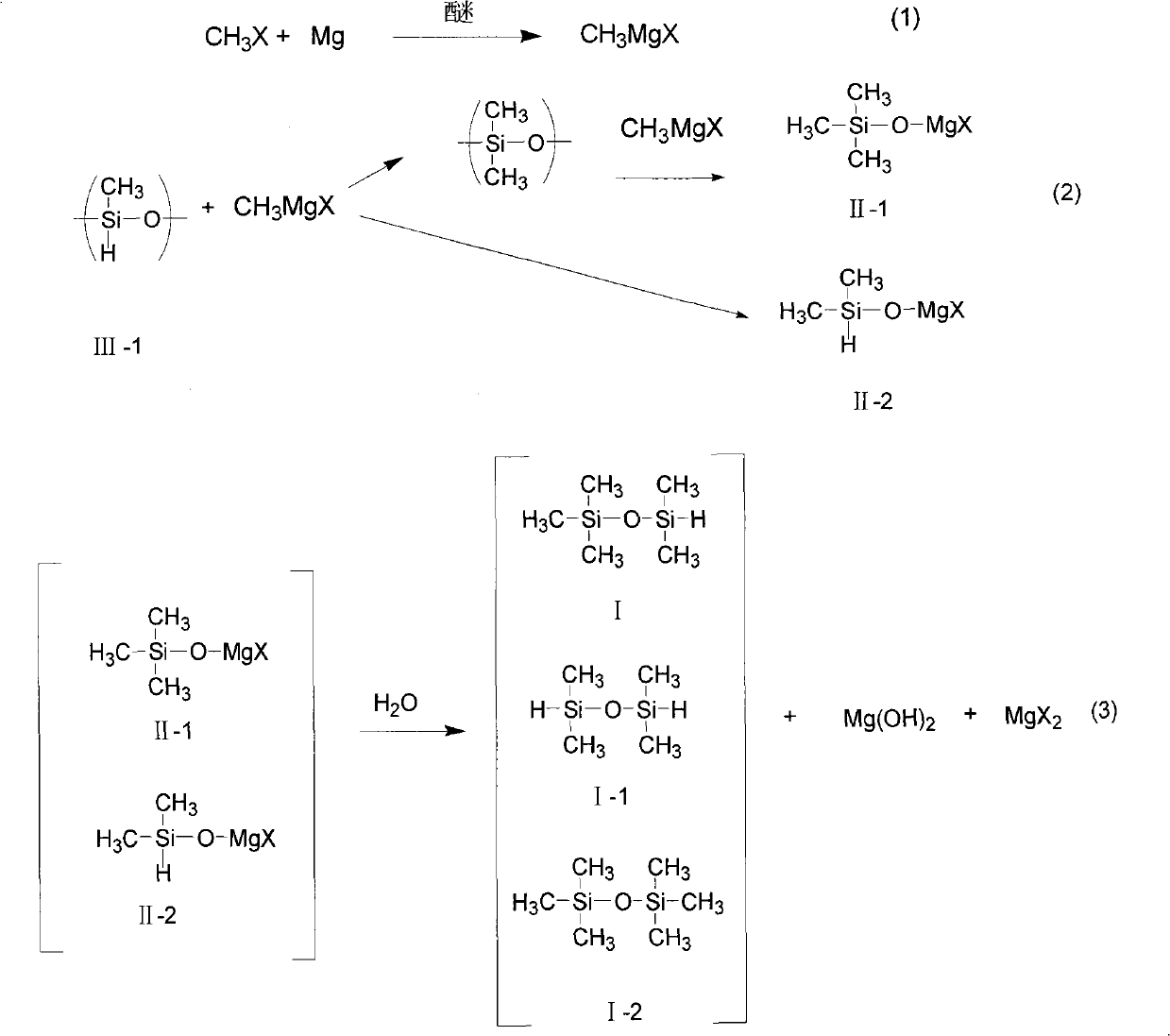
[0029] Add 7.2 grams (0.3 mol) of magnesium powder and 108 grams of ether into a 500 ml four-neck flask equipped with a thermometer, agitator, and condenser, and feed methyl bromide gas under nitrogen protection to control the reaction temperature at 50°C until the magnesium powder All gone. After reacting for 14 hours, 45 g (0.47 mol) of methyl bromide was introduced. Chemical analysis determined that the Grignard reagent content was 96%. Add 100 grams of toluene to the above-mentioned four-necked bottle, cool to about 20°C, add n=20 (molecular weight is about 1300), and 30 grams of high hydrogen-containing silicone oil (hydrogen content 1.56%) with n=20 (molecular weight is about 1300) and both ends are methyl groups (mainly The molar number of content fragments is 0.46 moles), and the reaction was carried out at 20° C. for 5 hours. Then, the temperature was controlled at 15° C., and 200 grams of 20% dilute sulfuric acid was added dropwise to the reactant in the above-ment...
Embodiment 2
[0033]Add 7.2 grams (0.3 mol) of magnesium powder and 108 grams of ether into a 500 ml four-necked bottle equipped with a thermometer, agitator, and condenser, and feed in methyl chloride gas under nitrogen protection, and control the reaction temperature at 50°C until the magnesium powder disappears completely. . The reaction time was 18 hours, 25 g (0.5 mol) of methyl chloride was introduced, and the content of the Grignard reagent was determined to be 95% by chemical analysis. Add 120 grams of toluene to the above-mentioned four-necked bottle, and add 38.4 grams of high hydrogen-containing silicone oil (hydrogen content 1.56%) with n=20 (molecular weight is about 1300) and methyl groups at both ends (main content fragment) dropwise at 30 ° C. The number of moles is 0.6 mol), and the reaction was incubated for 8 hours. Then, the temperature was lowered to 15° C., and 200 grams of 20% dilute sulfuric acid was added dropwise to the above-mentioned four-necked flask. After the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com