Polythiophene derivatives and synthesizing method thereof
A technology of polythiophene derivatives and synthesis methods, applied in the field of polythiophene derivatives and their synthesis, can solve the problems of low open circuit voltage, limit the improvement of energy conversion efficiency, etc., achieve mild polymerization conditions, reduce HOMO energy level, and simple polymerization method Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1 synthetic polymer P1
[0032] Process 2
[0033]
[0034] As shown in the above formula, the steps for preparing the polymer P1 are described. Add 1mmol of pre-polymer monomer S to a 100mL glass reaction bottle, then add 60mL of dry chloroform, stir and add 4mmol of anhydrous ferric chloride, react at room temperature for 24 hours, stop the reaction, pour into 200mL of methanol for precipitation, and pump The solid was obtained by filtration, stirred in aqueous ammonia for 3 hours, and then filtered with suction. Extract the polymer with methanol and n-hexane Soxhlet, dissolve the polymer with chloroform, and finally precipitate with methanol to obtain the purple polymer P1.
Embodiment 2
[0035] Embodiment 2 synthetic polymer P2-P7
[0036] P2-P7 According to the synthesis method of Flowchart 2, add 1mmol pre-polymer monomer to 100mL glass reaction bottle, then add 60mL dry chloroform, stir and add 4mmol anhydrous ferric chloride, react at room temperature for 24 hours, then stop the reaction , poured into 200mL of methanol for precipitation, filtered with suction to obtain a solid, stirred in aqueous ammonia for 3 hours, and filtered with suction again. Extract the polymer with methanol and n-hexane Soxhlet, dissolve the polymer with chloroform, and finally precipitate with methanol to obtain purple polymers (P2-P7).
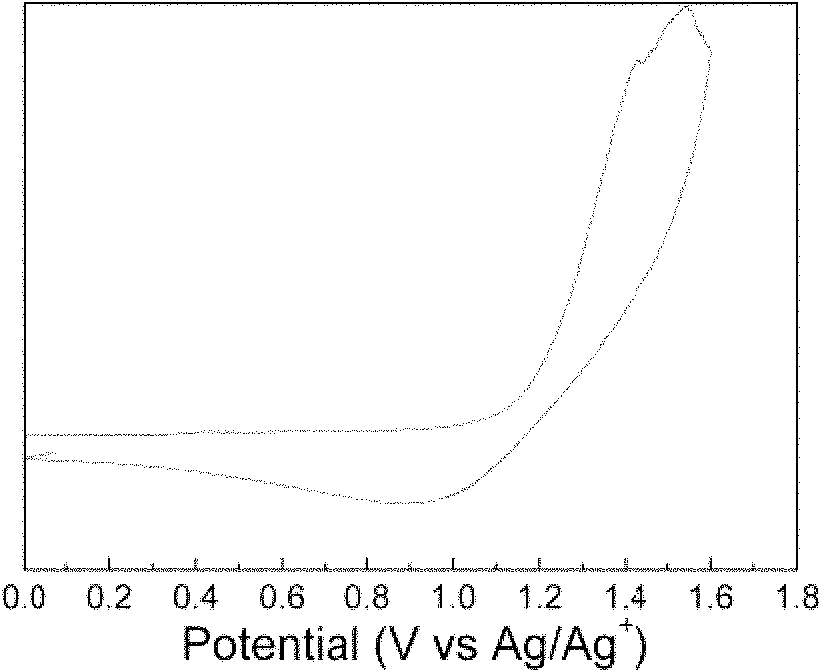
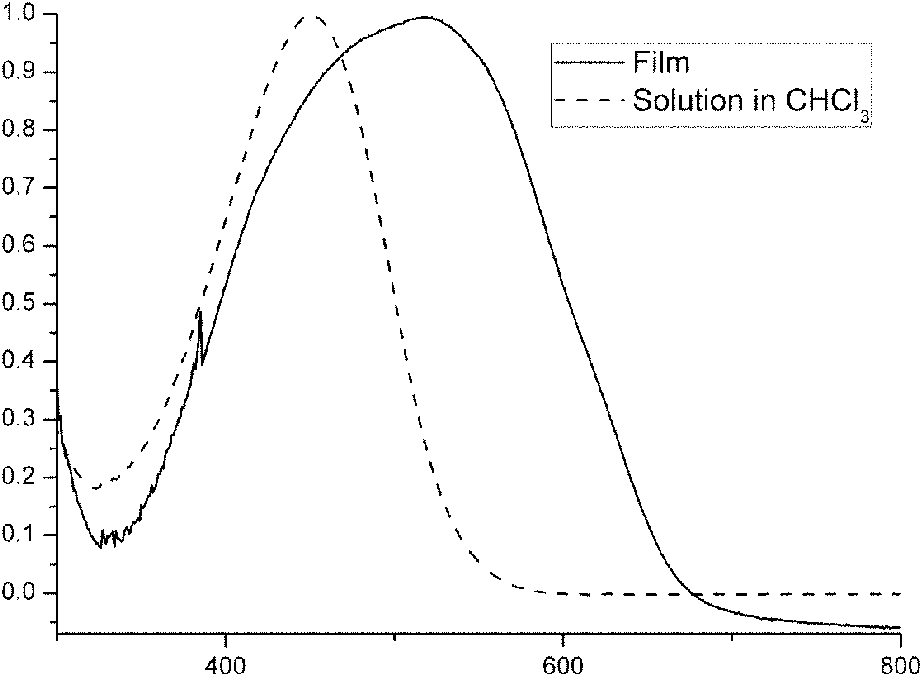
[0037] Such as figure 1 As shown, through the characterization of the polymer performance, the initial oxidation peak of the polymer is at 1.0V, which shows that the polymer has sufficient air stability, calculated by the formula: HOMO=-(E 氧化+4.71), the HOMO energy level of the available polymer is -5.71eV, which is 0.5eV lower than the report...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com