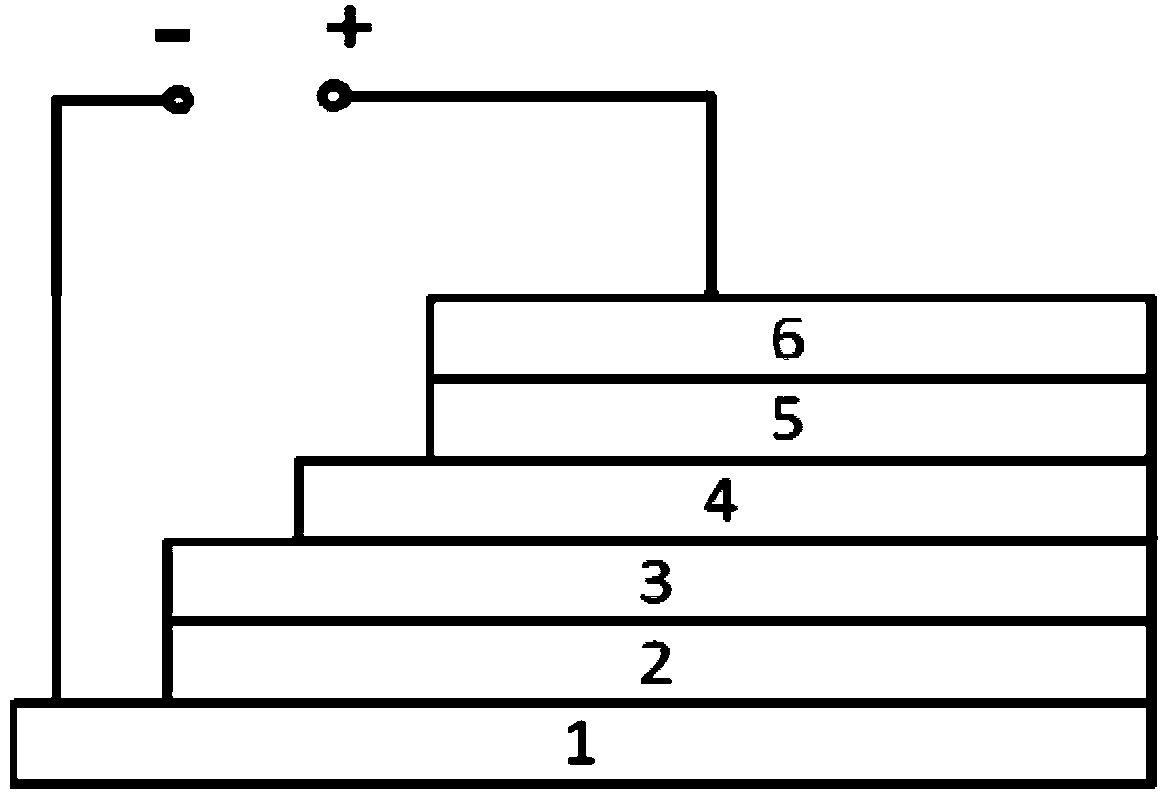
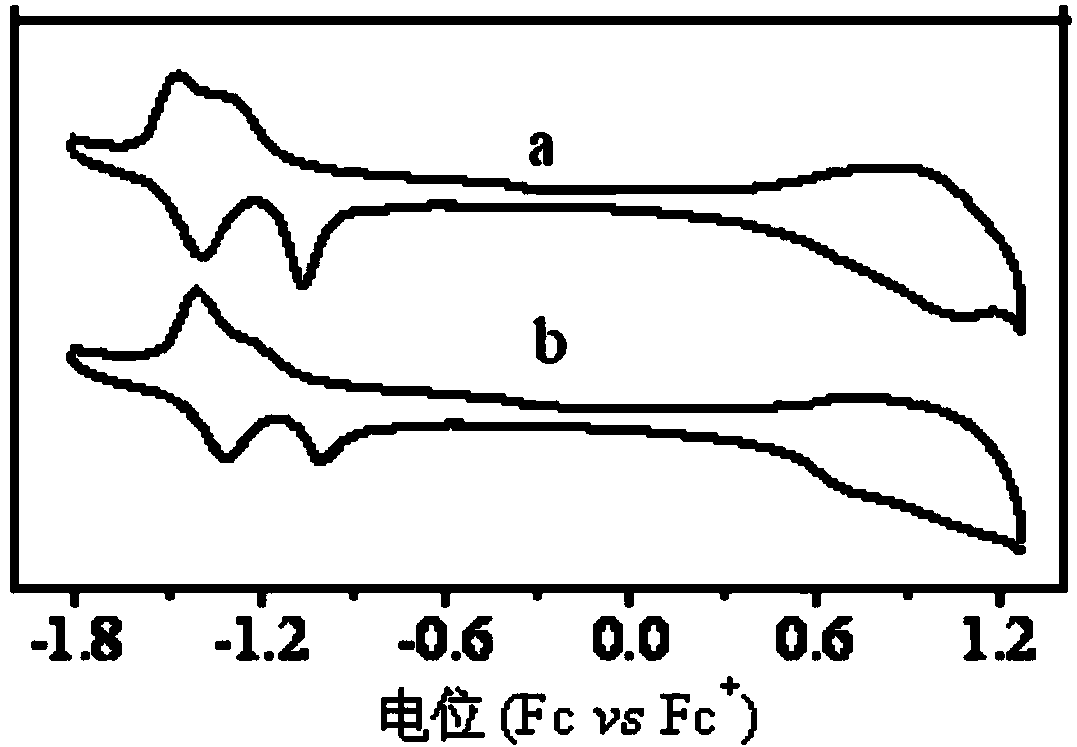
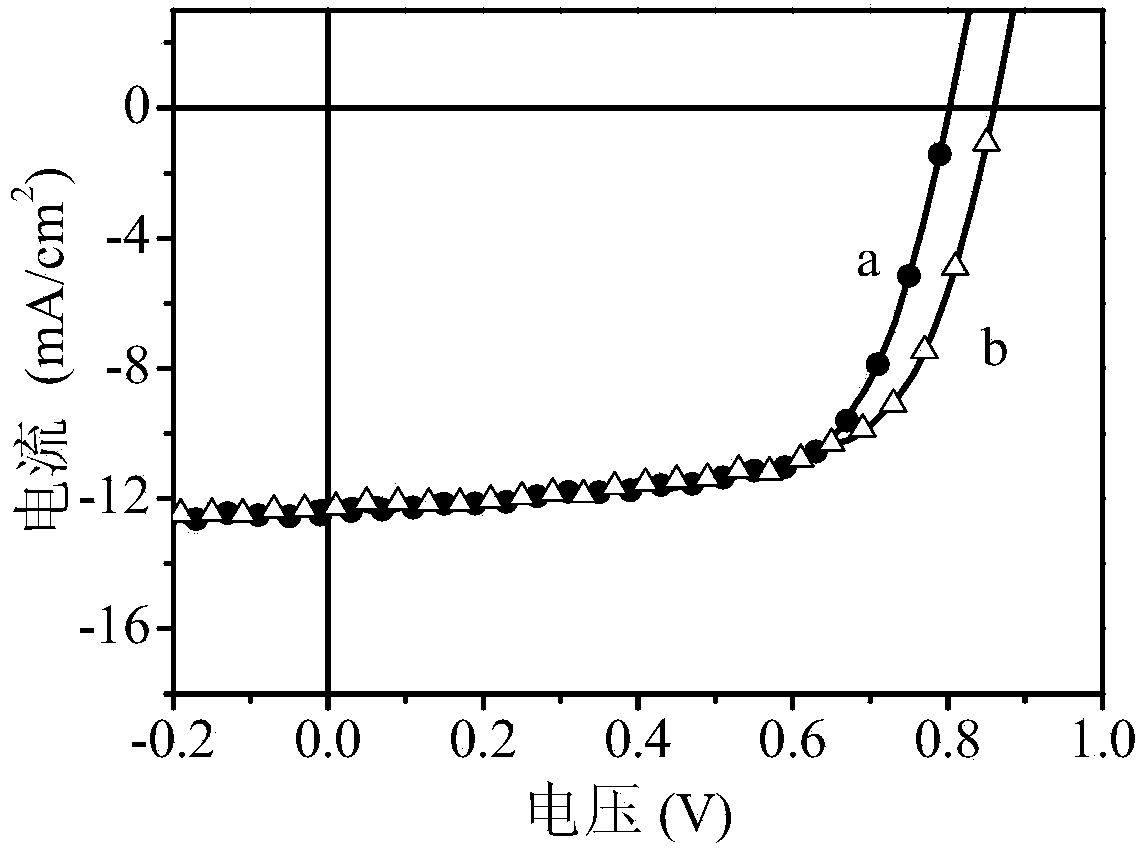
Polymer, preparation method thereof and organic polymer solar battery
A polymer and compound technology, applied in circuits, photovoltaic power generation, electrical components, etc., can solve problems such as low utilization rate, low battery efficiency, and narrow absorption band of conjugated compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] The present invention also provides a preparation method of a polymer represented by formula (I), comprising the following steps: under the condition of inert gas protection, combining a compound having a structure of formula (III-1) with a compound having a structure of formula (IV) The compound is mixed under the action of a catalyst and an auxiliary ligand, and reacted to obtain a polymer represented by formula (I);
[0037]
[0038] Among them, R 1 is an alkyl group; R 2 Is an alkyl group; X is H or a fluorine atom; n is the degree of polymerization.
[0039] In the present invention, the R 1 , R 2 , X and n are the same as above, and will not be repeated here.
[0040] The present invention has no special limitation on the sources of all raw materials, which can be commercially available or self-made.
[0041] The compound with the structure of formula (IV) is preferably prepared according to the following method: S1) The compound with the structure of form...
Embodiment 1
[0062] 1.1 Under argon atmosphere, add 16.39mmol 6-bromoindol-2-one and 16.39mmol 6-bromo-7-fluoroisatin to a 250ml three-necked flask, add 200ml glacial acetic acid and 1.2ml concentrated hydrochloric acid, and heat to 130 React at ℃ for 24 hours, cool to room temperature, filter, wash with water, ethanol, and ether in turn, and the red solid that is drained is 6,6′-dibromo-7-fluoroisoindigo, with a yield of 70%.
[0063] 1.2 Dissolve 2.28mmol of 6,6′-dibromo-7-fluoroisoindigo obtained in 1.1 and 9.13mmol of potassium hydroxide in 40ml of DMF, and add 9.13mmol of 1-iodo-2- Octyldodecane and 40ml THF were slowly dropped into the reaction solution, and after 24 hours of reaction at room temperature, poured into water, extracted with chloroform, and dried the organic layer with anhydrous magnesium sulfate. After washing with petroleum ether and dichloromethane as the eluent, the red solid is N,N'-bis(2-octyldodecyl)-6,6'-dibromo-7-fluoroisoindigo, Yield 42%.
[0064] 1.3 Under...
Embodiment 2
[0073] 2.1 Under argon atmosphere, add 16.39mmol 6-bromoindol-2-one and 16.39mmol 6-bromo-7-fluoroisatin into a 250ml three-necked flask, add 200ml glacial acetic acid and 1.2ml concentrated hydrochloric acid, heat to 130 React at ℃ for 24 hours, cool to room temperature, filter, wash with water, ethanol, and ether in turn, and the red solid that is drained is 6,6′-dibromo-7-fluoroisoindigo, with a yield of 70%.
[0074] 2.2 Dissolve 2.28mmol of 6,6′-dibromo-7-fluoroisoindigo obtained in 2.1 and 9.13mmol of potassium hydroxide in 40ml of DMF, and add 9.13mmol of 1-iodo-2- Butyl octane and 40ml THF were slowly dripped into the reaction liquid, and after reacting at room temperature for 24 hours, they were poured into water, extracted with chloroform, and the organic layer was dried over anhydrous magnesium sulfate. After rinsing with ether and dichloromethane as the eluent, a red solid was obtained which was N,N'-bis(2-butyloctyl)-6,6'-dibromo-7-fluoroisoindigo, and the yield w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com