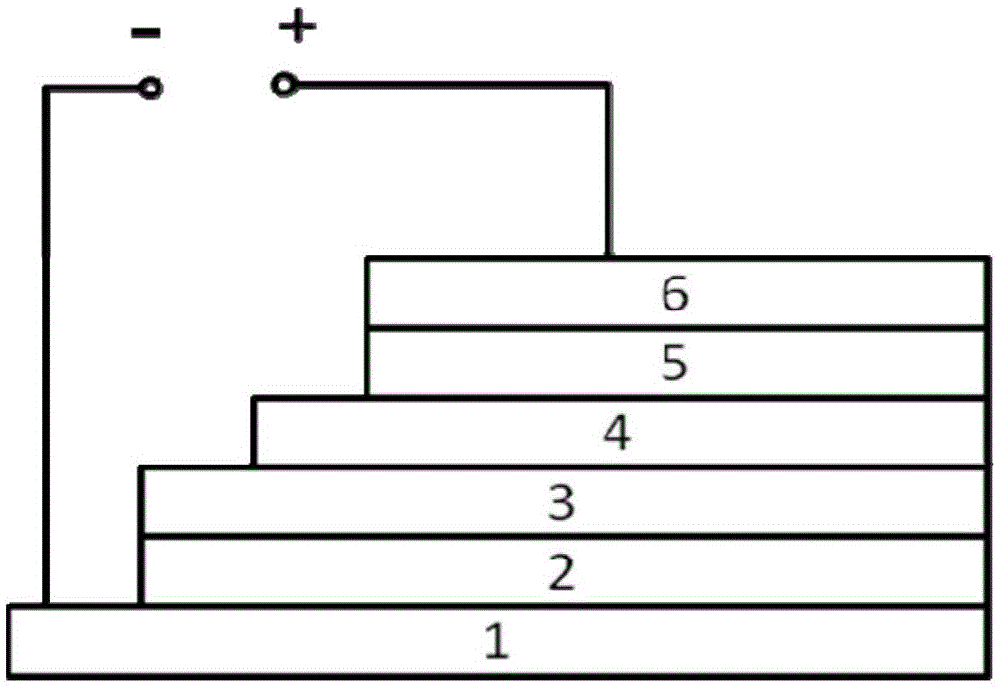
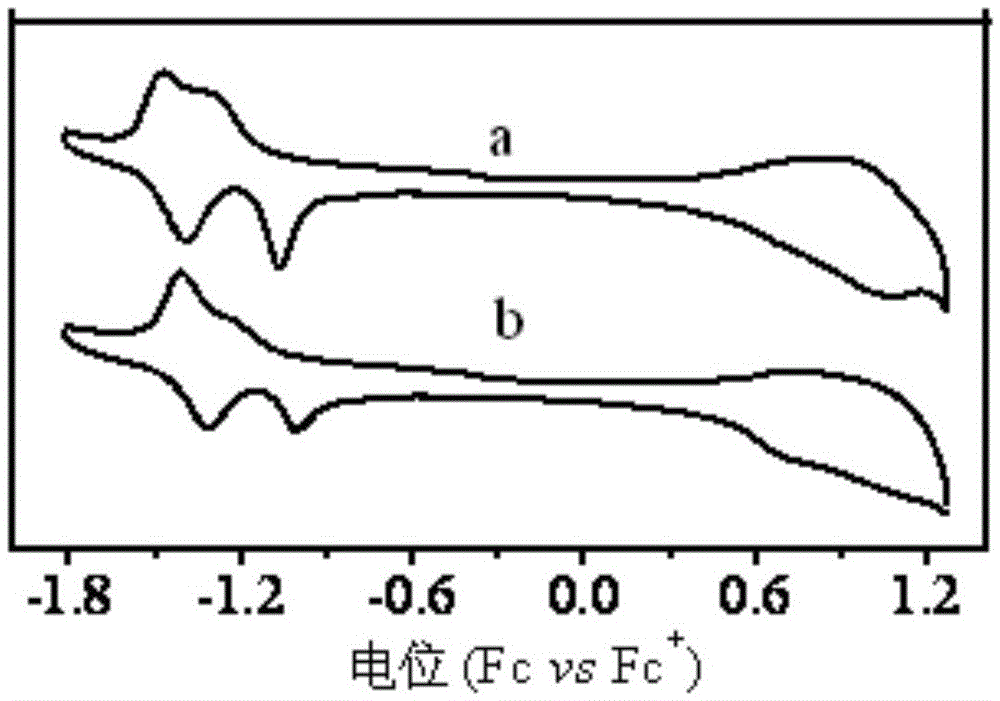
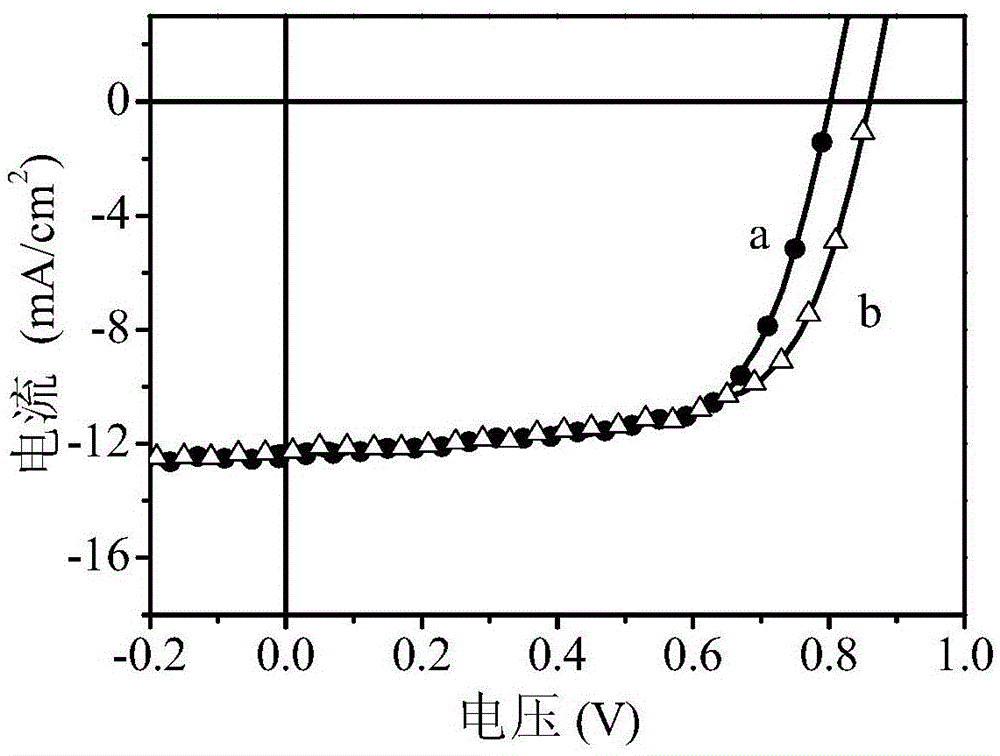
Polymer and preparation method thereof, organic polymer solar cell
A polymer and compound technology, applied in circuits, photovoltaic power generation, electrical components, etc., can solve problems such as low utilization rate, ineffective use of solar spectrum, and low hole mobility of conjugated polymers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] The present invention also provides a preparation method of a polymer represented by formula (I), comprising the following steps: under the condition of inert gas protection, combining a compound having a structure of formula (III-1) with a compound having a structure of formula (IV) The compound is mixed under the action of a catalyst and an auxiliary ligand, and reacted to obtain a polymer represented by formula (I);
[0037]
[0038] Among them, R 1 is an alkyl group; R 2 Is an alkyl group; X is H or a fluorine atom; n is the degree of polymerization.
[0039] In the present invention, the R 1 , R 2 , X and n are the same as above, and will not be repeated here.
[0040] The present invention has no special limitation on the sources of all raw materials, which can be commercially available or self-made.
[0041] The compound with the structure of formula (IV) is preferably prepared according to the following method: S1) The compound with the structure of form...
Embodiment 1
[0062] 1.1 Under argon atmosphere, add 16.39mmol 6-bromoindol-2-one and 16.39mmol 6-bromo-7-fluoroisatin into a 250ml three-necked flask, add 200ml glacial acetic acid and 1.2ml concentrated hydrochloric acid, and heat to 130°C for reaction After 24 hours, cool to room temperature, filter, and wash with water, ethanol, and ether in sequence. The dried red solid is 6,6′-dibromo-7-fluoroisoindigo, and the yield is 70%.
[0063] 1.2 Dissolve 2.28 mmol of 6,6′-dibromo-7-fluoroisoindigo and 9.13 mmol of potassium hydroxide in 40 ml of DMF, and add 9.13 mmol of 1-iodo-2- Octyldodecane and 40ml THF were slowly dropped into the reaction solution, and after reacting at room temperature for 24 hours, poured into water, extracted with chloroform, dried the organic layer with anhydrous magnesium sulfate, removed the solvent, and mixed with petroleum at a volume ratio of 8:1 After rinsing with ether and dichloromethane as the eluent, the red solid is N,N'-bis(2-octyldodecyl)-6,6'-dibromo-7...
Embodiment 2
[0073] 2.1 Under argon atmosphere, add 16.39mmol 6-bromoindol-2-one and 16.39mmol 6-bromo-7-fluoroisatin into a 250ml three-necked flask, add 200ml glacial acetic acid and 1.2ml concentrated hydrochloric acid, and heat to 130°C for reaction After 24 hours, cool to room temperature, filter, and wash with water, ethanol, and ether in sequence. The dried red solid is 6,6′-dibromo-7-fluoroisoindigo, and the yield is 70%.
[0074] 2.2 Dissolve 2.28 mmol of 6,6′-dibromo-7-fluoroisoindigo and 9.13 mmol of potassium hydroxide in 40 ml of DMF, and add 9.13 mmol of 1-iodo-2- Butyl octane and 40mlTHF were slowly dripped into the reaction solution, and after 24 hours of reaction at room temperature, poured into water, extracted with chloroform, dried the organic layer over anhydrous magnesium sulfate, and removed the solvent, and mixed petroleum ether with a volume ratio of 8:1 After rinsing with dichloromethane as eluent, the red solid is N,N'-bis(2-butyloctyl)-6,6'-dibromo-7-fluoroisoin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com