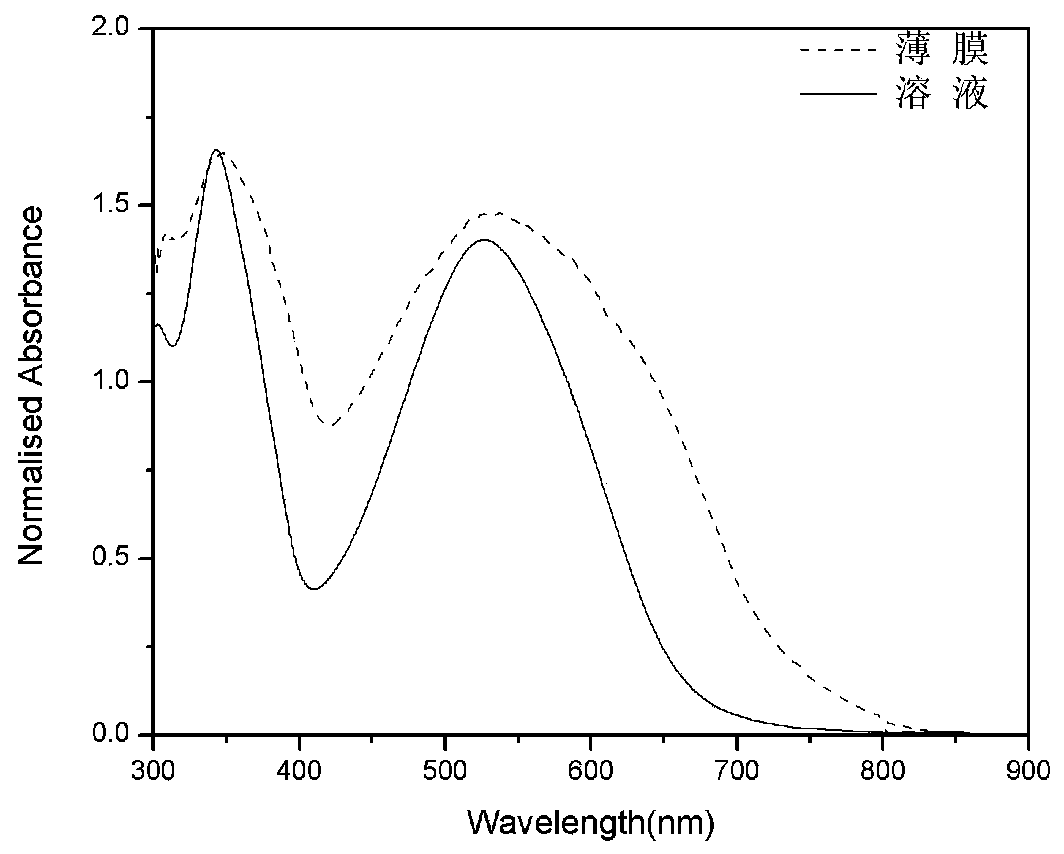
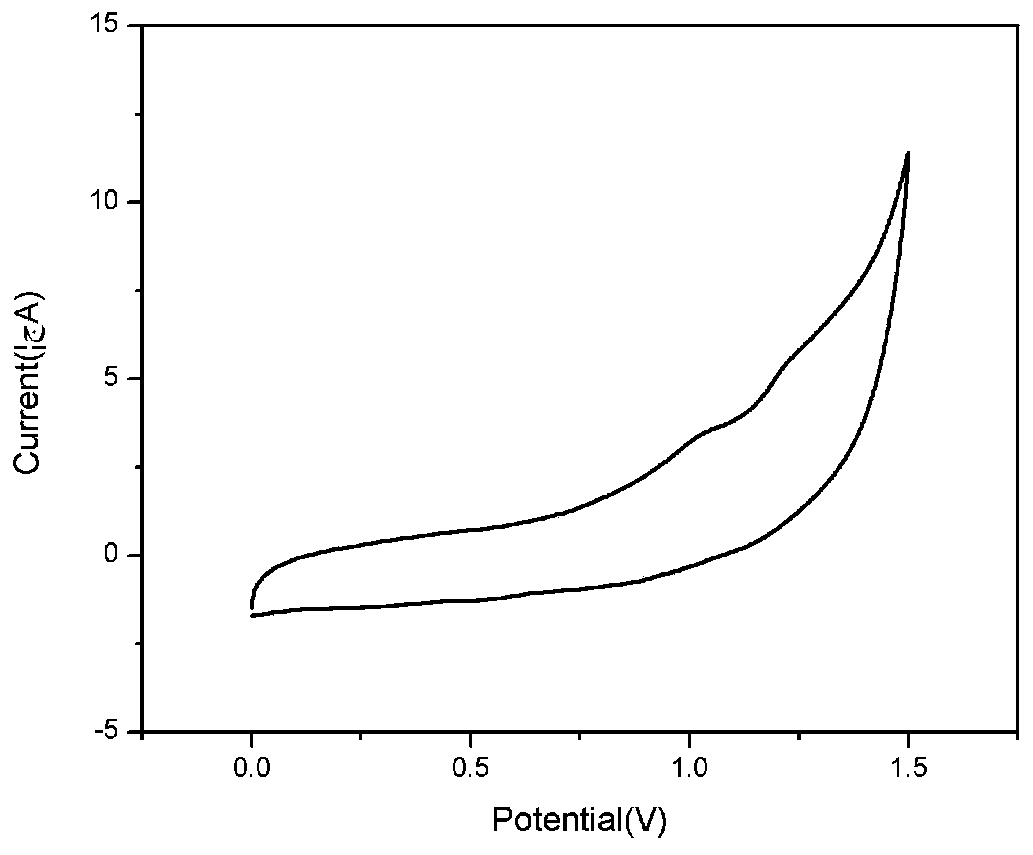
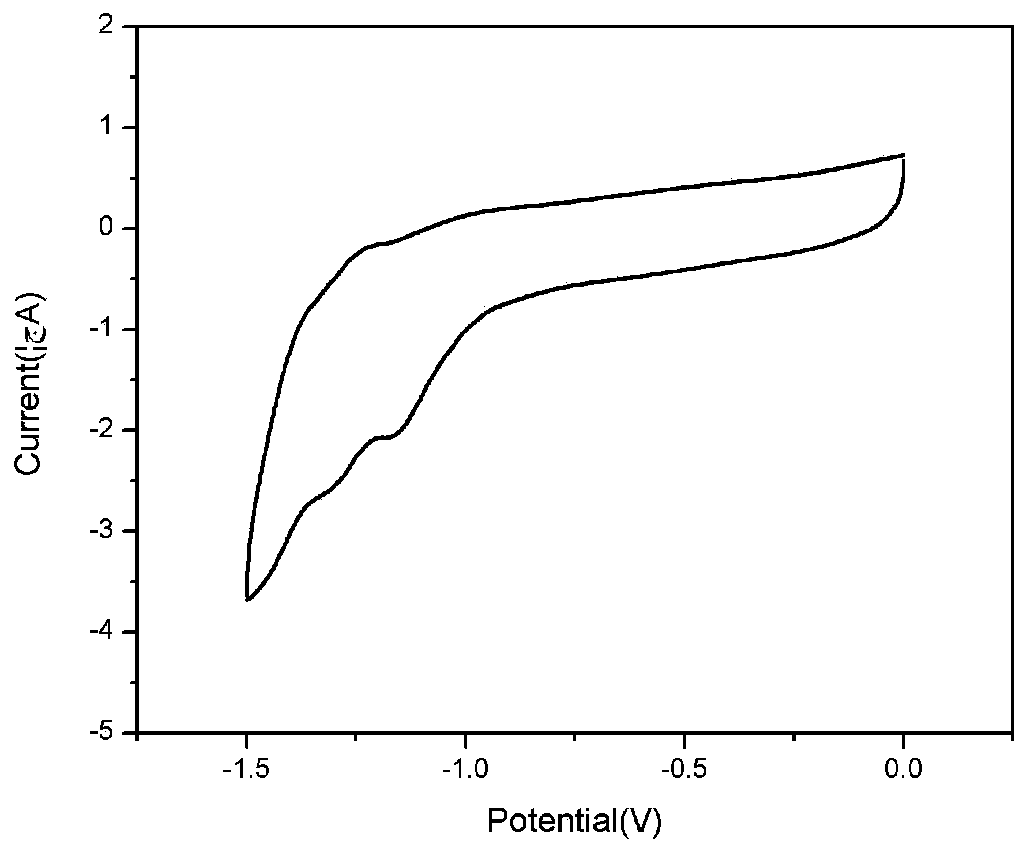
Donor-acceptor-donor-acceptor-donor oligothiophene derivative taking dithienopyrrole as molecular center and preparation method of derivative
A technology of thiophene derivatives and dithiophene, which is used in semiconductor/solid-state device manufacturing, photovoltaic power generation, electrical components, etc., can solve problems such as difficulty in practical application, poor solubility and film-forming properties, and difficulty in making devices, etc. Achieve the effect of narrow band gap, low HOMO energy level and clear chemical structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] 2,6-Di{(4-(7-bromo-[1,2,5]thiadiazolo[3,4-c]pyridine)}-N-isooctyldithieno[3,2- b: Synthesis of 2',3'-d]pyrrole:
[0034] Under nitrogen protection, 2,6-bis(trimethyltin)-N-isooctyldithieno[3,2-b:2',3'-d]pyrrole (5.56g, 9mmol), 4,7-Dibromo-[1,2,5]thiadiazolo[3,4-c]pyridine (2.65g, 9mmol) and tetrakis(triphenylphosphine)palladium (0.15g, 0.13mmol) were added to 500ml Into a three-neck round bottom flask, inject 150ml of dry N,N-dimethylformamide, and stir the reaction at 80°C for 60h; after the reaction, cool the reactant to room temperature, add deionized water, extract three times with dichloromethane, and combine organic phase; the organic phase was washed with anhydrous MgSO 4 Dry, filter and concentrate under reduced pressure to obtain a small amount of dark red solid; the product is purified by silica gel column chromatography, the eluent is a mixture of dichloromethane and n-hexane (volume ratio 1:1), and a purple-red solid is obtained. Solid 0.43 g, yield 15.1%...
Embodiment 2
[0038] 2,6-Di{(4-(7-bromo-[1,2,5]thiadiazolo[3,4-c]pyridine)}-N-isooctyldithieno[3,2- b: Synthesis of 2',3'-d]pyrrole:
[0039] Under argon protection, 2,6-bis(trimethyltin)-N-isooctyldithieno[3,2-b:2',3'-d]pyrrole (2.16g, 3.5mmol ), 4,7-dibromo-[1,2,5]thiadiazolo[3,4-c]pyridine (2.58g, 8.75mmol) and bis(triphenylphosphine)palladium dichloride (0.12g ,0.17mmol) into a 500ml three-necked round-bottomed flask, inject 150ml of dry N,N-dimethylformamide, stir and react at 100°C for 35h; Chloromethane was extracted three times, and the organic phase was combined; the organic phase was washed with anhydrous MgSO 4Dry, filter and concentrate under reduced pressure to obtain a small amount of dark red solid; the product is purified by silica gel column chromatography, the eluent is a mixture of dichloromethane and n-hexane (volume ratio 1:1), and a purple-red solid is obtained. Solid 1.0 g, yield 39.8%.
[0040] Synthesis of oligomers:
[0041] Under the protection of argon, the ...
Embodiment 3
[0043] 2,6-Di{(4-(7-bromo-[1,2,5]thiadiazolo[3,4-c]pyridine)}-N-isooctyldithieno[3,2- b: Synthesis of 2',3'-d]pyrrole:
[0044] Under the protection of helium, 2,6-bis(trimethyltin)-N-isooctyldithieno[3,2-b:2',3'-d]pyrrole (3.01g, 4.9mmol ), 4,7-dibromo-[1,2,5]thiadiazolo[3,4-c]pyridine (7.21g, 24.50mmol) and tris(dibenzylideneacetone)dipalladium (0.12g, 0.13mmol) into a 500ml three-necked round-bottomed flask, inject 150ml of dry N,N-dimethylformamide, stir and react at 130°C for 10h; Extracted three times with methane, combined the organic phase; the organic phase was washed with anhydrous MgSO 4 Dry, filter and concentrate under reduced pressure to obtain a small amount of dark red solid; the product is purified by silica gel column chromatography, the eluent is a mixture of dichloromethane and n-hexane (volume ratio 1:1), and a purple-red solid is obtained. Solid 1.04 g, yield 30.1%.
[0045] Synthesis of oligomers:
[0046] Under the protection of helium, the interme...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com