Stable tegafur injection formula and preparation process thereof
A technology for tegafur and injection, which is applied in the field of stable tegafur injection formulation and preparation technology, can solve the problems of inconvenience to patients, decreased content of main drug, decreased pH value of finished products, etc., and achieves improved production capacity, increased safety Sexual protection, the effect of reducing the content of related substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add 200g of tegafur into 1000ml of 1mol / L carbonate buffer solution, mix and stir for 10 minutes until completely dissolved, add 3500ml of water for injection, adjust the pH value to 9.6 with 1mol / L sodium hydroxide solution or 1M hydrochloric acid, Water for injection was added to make the volume to 5000ml, and the solution was sterilized and filtered with a 0.22 μm filter membrane until it became clear. After passing the test, it was filled into 1000 5ml ampoules to prepare the finished tegafur injection.
Embodiment 2
[0024] Add 200g of tegafur into 1000ml of 1mol / L carbonate buffer, heat to 40±2°C, mix and stir for 10 minutes until completely dissolved, add 3500ml of water for injection, and use 1mol / L sodium hydroxide solution or 1M Adjust the pH value to 10.0 with hydrochloric acid, add water for injection to make up to 5000ml, sterilize and filter the solution with a 0.22μm filter membrane until clear, fill it into 1000 5ml ampoules after passing the test, and prepare tegafur injection finished product.
Embodiment 3
[0026] Add 200g of tegafur into 1000ml of 1mol / L carbonate buffer, heat to 40±2°C, mix and stir for 10 minutes until completely dissolved, add 3500ml of water for injection, and use 1mol / L sodium hydroxide solution or 1M Adjust the pH value to 10.0 with hydrochloric acid, and add water for injection to make the volume to 5000ml. Add activated carbon to adsorb the solution, and filter it with a 0.45 μm filter membrane after 30 minutes until it becomes clear. Then the solution was sterilized and filtered with a 0.22 μm filter membrane until it became clear, and after passing the test, it was filled into 1000 5ml ampoules to obtain the finished product of tegafur injection.
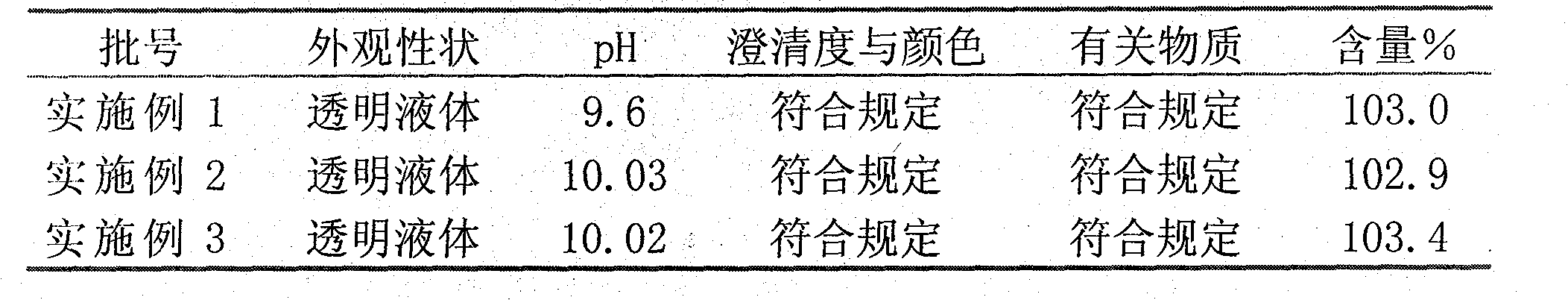
[0027] Example 1-3 Stability investigation, investigation conditions: 25±2°C, 90 days
[0028]
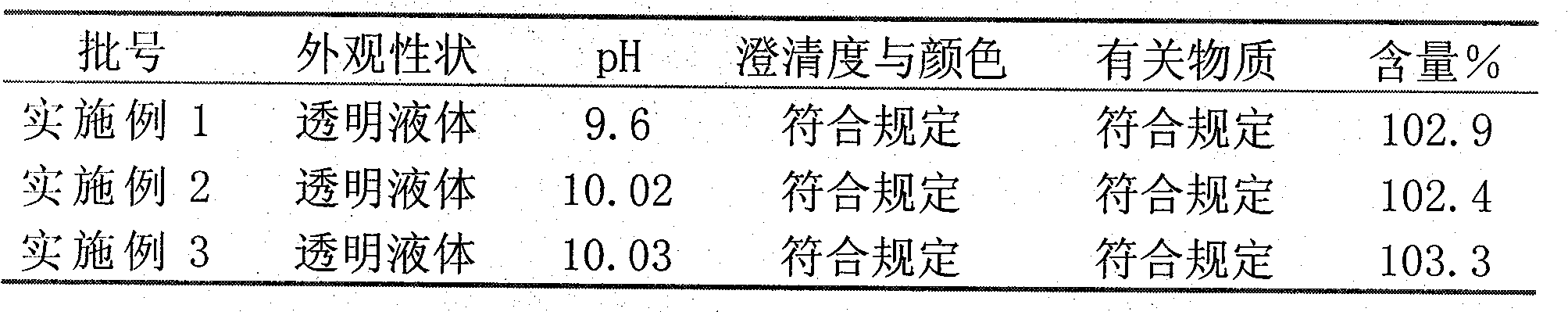
[0029] Example 1-3 Stability investigation, investigation conditions: 25±2°C, 180 days
[0030]
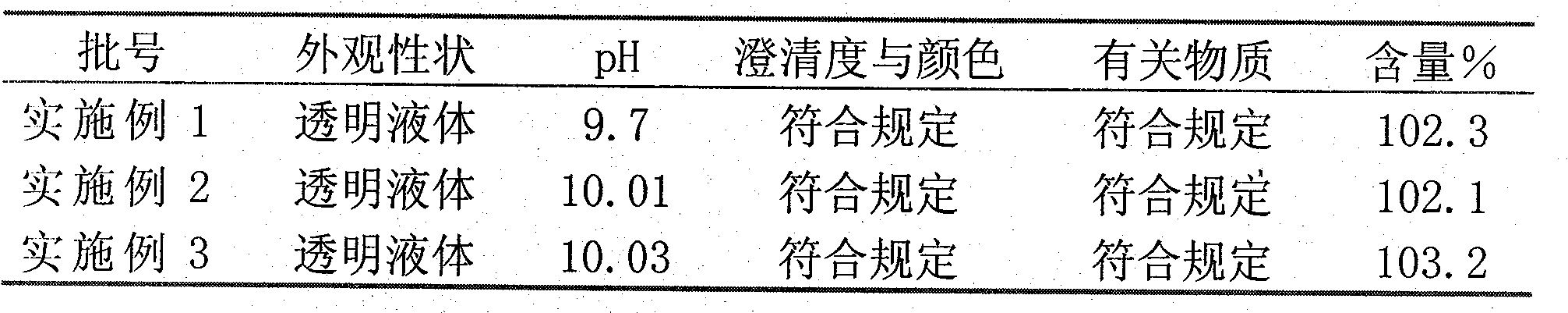
[0031] Example 1-3 Stability investigation, investigation conditions: 25±2°C, 360 days
[0032]
[003...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com