Oxaliplatin liposome, preparation method and application thereof
A technology of oxaliplatin and liposomes, which is applied in the field of oxaliplatin liposomes and its preparation, can solve the problems of poor popularity, harsh conditions in the fusion process of reversed micelles and blank liposome membranes, and Issues such as low sealing rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
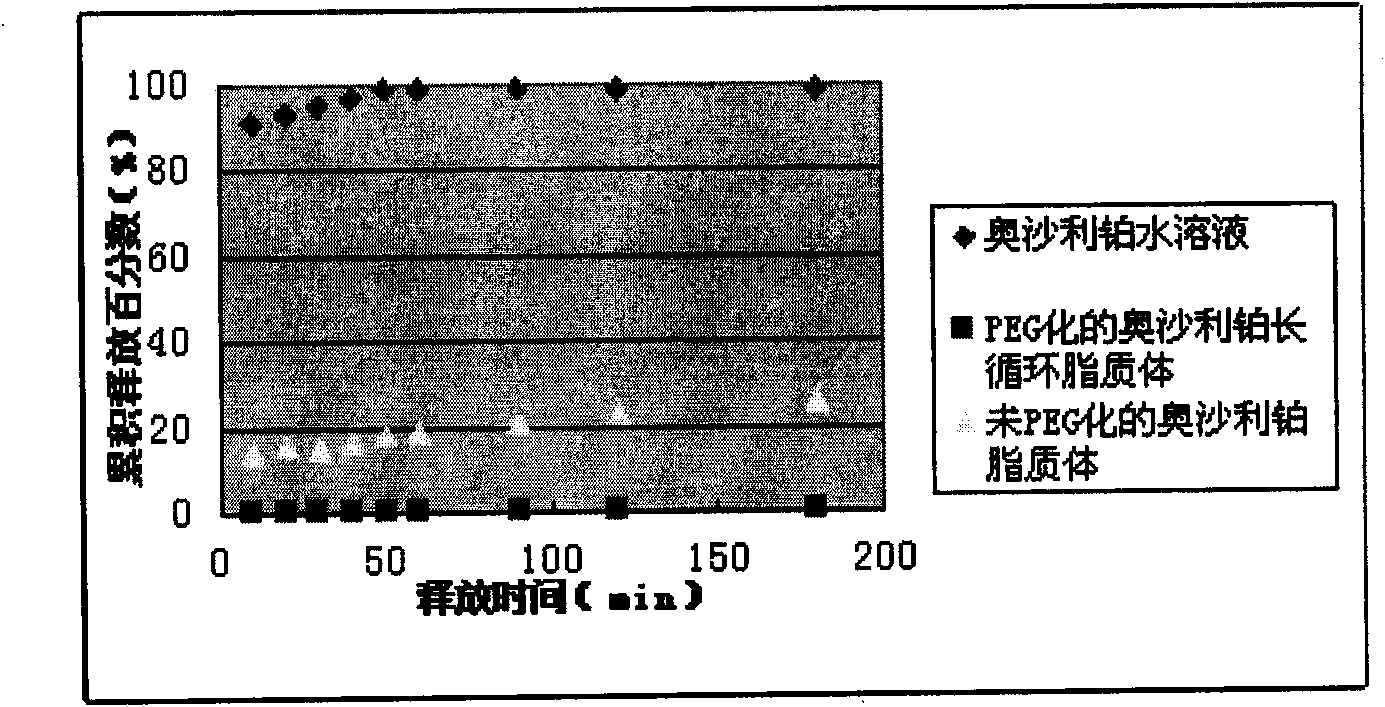
Image
Examples
preparation example Construction
[0058] The invention provides a method for preparing oxaliplatin liposomes, which is to make the reverse micelles spontaneously transform into liposomes by removing the organic solvent in the reverse micelles composition on the basis of preparing the reverse micelles composition.
[0059] The preparation method of reverse micelles comprises the following steps:
[0060] (1) mixing cholesterol, phospholipids and an organic solvent to obtain an organic phase;
[0061] (2) Oxaliplatin and buffer are mixed to obtain an aqueous phase; and
[0062] (3) Mix the organic phase and the aqueous phase obtained in steps (1) and (2) to obtain reversed-phase micelles.
[0063] The weight ratio of oxaliplatin and phospholipid is 1:3-20; the weight ratio of cholesterol and phospholipid is 1:2-10; the organic solvent is selected from chloroform, ether, or a mixture thereof; the mixture of chloroform and ether The volume ratio of the two is 1:4-4:1.
[0064] The buffer solution has a pH of 5....
Embodiment 1
[0087] Preparation of stable reverse micelles I
[0088]
[0089] cholesterol
[0090] Preparation method: Take natural egg yolk phospholipids (purity is 90%) and cholesterol according to the prescription amount, place them in an eggplant-shaped bottle, add 10ml of chloroform / ether mixed solvent (4:1, v / v) to dissolve them; weigh 4.0 mg oxaliplatin was dissolved in 1 ml of acetic acid-sodium acetate buffer (0.05M, pH 5.0), and then added to the lipid solution. After vortex mixing, short-term ultrasonication in an ice bath forms a stable reversed-phase micelle I, which exists stably at room temperature for more than 30 minutes.
Embodiment 2
[0092] Preparation of stable reverse micelles II
[0093] Oxaliplatin
[0094] Preparation method: Take natural egg yolk phospholipids (purity is 75%) and cholesterol according to the prescription, place them in eggplant-shaped bottles, add 3ml of chloroform / ether mixed solvent (1:4, v / v) to dissolve them; weigh 8.0 mg oxaliplatin was dissolved in 1 ml of citric acid-sodium citrate buffer (1M, pH 6.2), and then added to the lipid solution. After vortex mixing, short-time ice-bath sonication forms a stable reversed-phase micelle II, which exists stably at room temperature for more than 30 minutes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com