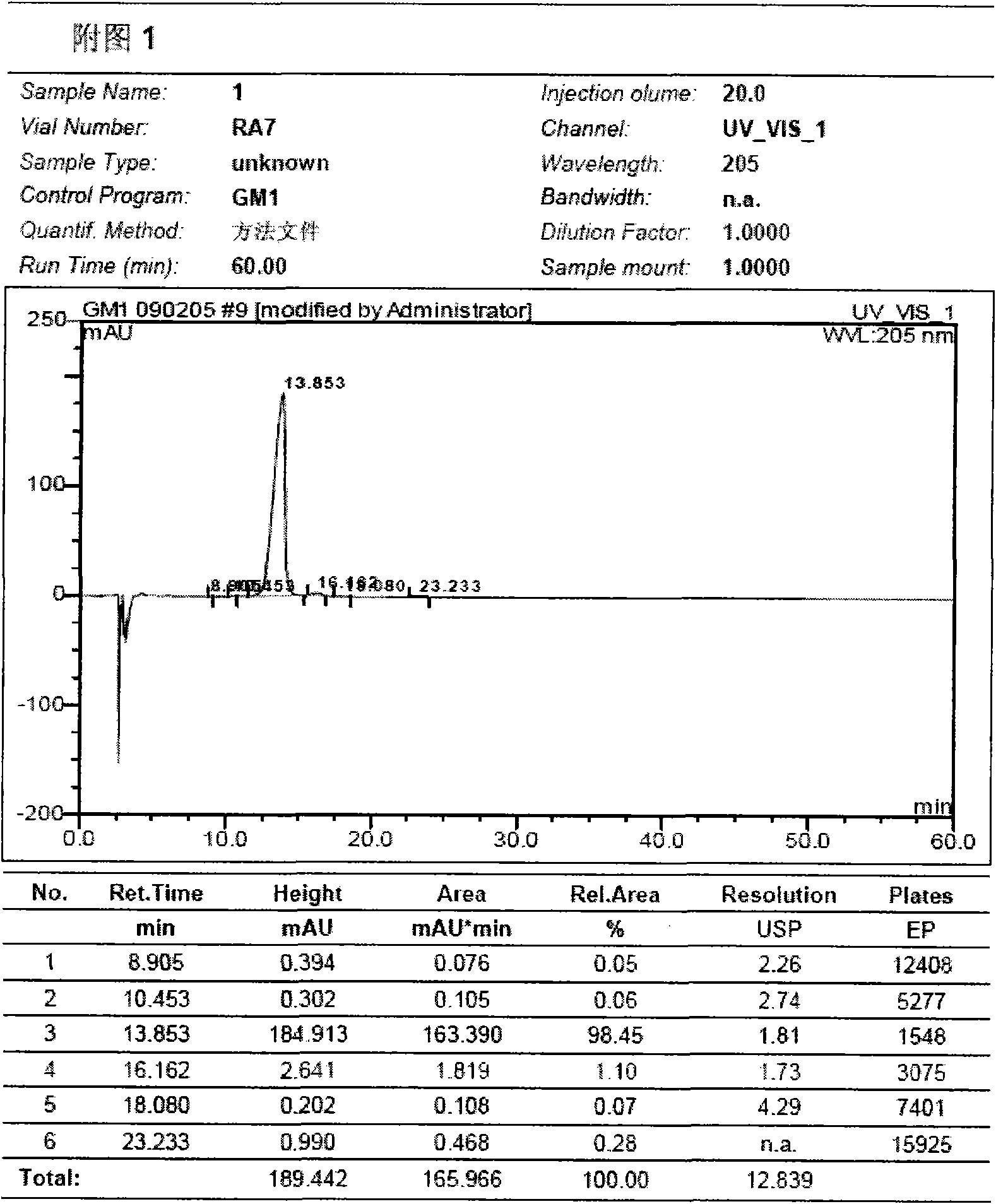
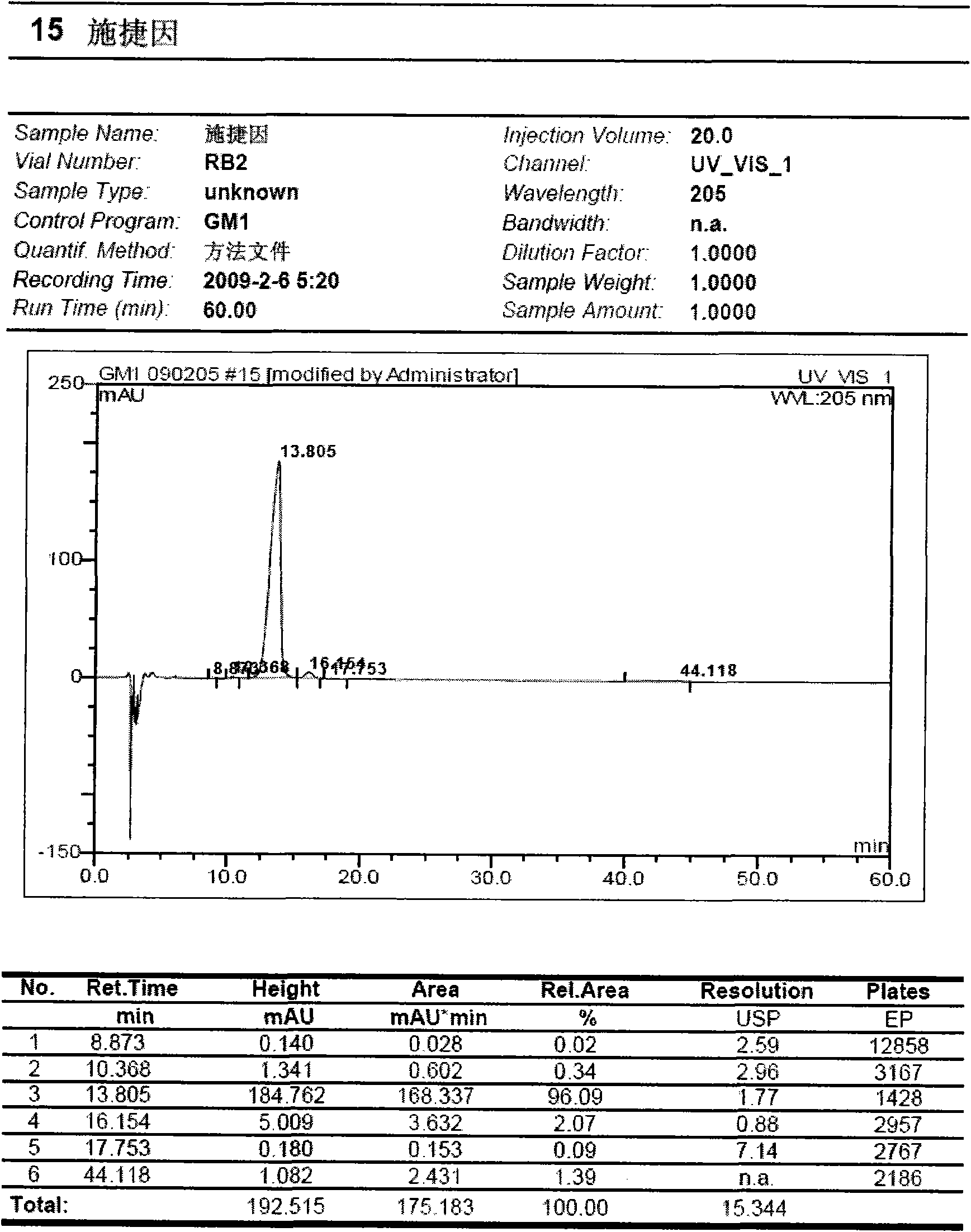
Preparation method for monosialotetrahexosyl ganglioside and monosialotetrahexosyl ganglioside sodium injection or freeze-dried powder injection
A technology of ganglioside and monosialic acid, which is applied in the field of biological drug preparation, can solve problems such as low toxicity, and achieve the effects of increasing content, improving safety, and reducing the content of impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Take pig brain tissue, weigh it after removing impurities, add the same amount of purified water to prepare homogenate, add 2 times the amount (V / V) of 2% Triton X-100 solution to the homogenate, stir and extract at 4-20°C for 15 hours, and centrifuge to remove Precipitation, the precipitation was extracted once again with 2% Triton X-100 solution of 2 times the amount (W / W), the extraction time was 5 hours, the extracts were combined, concentrated with an ultrafiltration membrane with a molecular weight cut-off of 10KD, and concentrated to the original volume About one-fifth of the ganglioside crude extract was obtained. Calculated by the amount of sialic acid, the content of ganglioside is 0.83mg / ml.
Embodiment 2
[0059] Take the bovine brain tissue, weigh it after removing impurities, add an equal amount of purified water to prepare a homogenate, use 1 times the amount (V / V) of 5% Tween-80 solution for the homogenate, stir and extract at 40°C for 10 hours, filter to remove the precipitate, The precipitate was extracted once again with 1 times the amount (W / W) of 5% Tween-80 solution, and the extraction time was 4 hours. The extracts were combined and concentrated under reduced pressure to about one-eighth of the original volume to obtain crude gangliosides. Extraction. Calculated by the amount of sialic acid, the content of ganglioside is 3.02mg / ml.
Embodiment 3
[0061] Take the pig brain tissue, weigh it after removing impurities, add the same amount of purified water to prepare homogenate, use 4 times the volume (V / V) of 1% Brij35 solution for the homogenate, stir and extract at 60°C for 4 hours, and centrifuge to remove the precipitate, precipitate Extract again 2 times with 1% Brij35 solution of 2 times the amount (W / W), the extraction time is 2 hours and 1 hour respectively, the combined extracts are concentrated with an ultrafiltration membrane with a molecular weight cut-off of 10KD, to four times the original volume. About one-third, to obtain the crude extract of gangliosides. Calculated by the amount of sialic acid, the content of ganglioside is 0.31mg / ml.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com