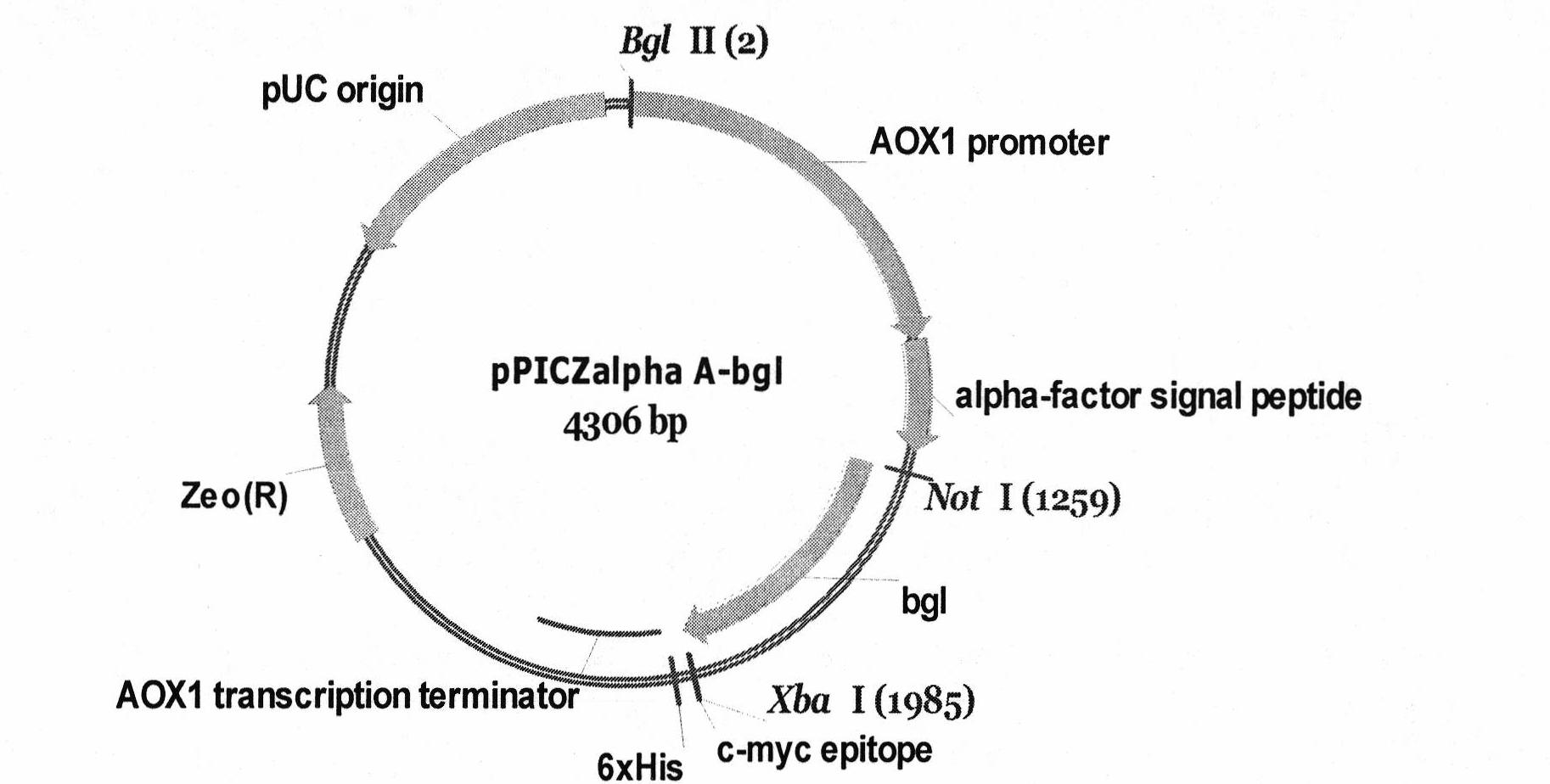
High-yield and temperature-resistant beta-dextranase pichia pastoris and construction thereof
The technology of glucanase and Pichia pastoris is applied in the field of high-yield and temperature-tolerant beta-glucanase Pichia pastoris and its construction, which can solve foggy turbidity, gel precipitation, and reduce abiotic stability of beer. issues of sex
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
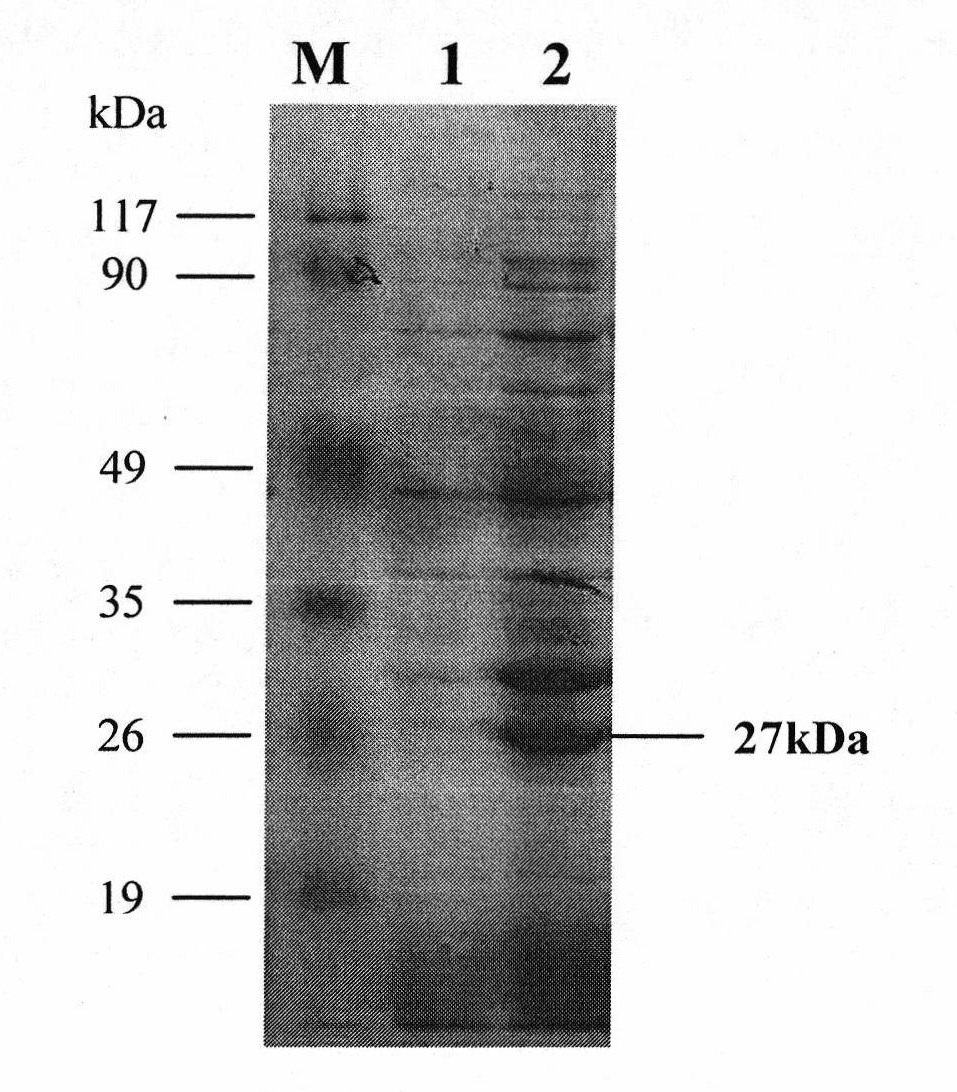
[0028] The recombinant Pichia pastoris GS115-pPICZαA-bgl obtained by the invention was induced to produce enzymes in the Mut+ induction mode, and the optimal production of β-1,3-1,4-glucanase was obtained by optimizing the culture conditions of the recombinant strains. The best expression conditions are: pH 7.0, OD 600 2.5, the daily induction addition amount of methanol is 1%, and the culture time of the thalline after methanol induction is 2.5-3 days. Under these conditions, the protein expression of β-glucanase secreted into the culture medium was 190mg / L, and the specific enzyme activity reached 4312U / mg protein. SDS-PAGE results showed that the size of the expressed protein was about 27KDa, which was consistent with the theoretical molecular weight.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com