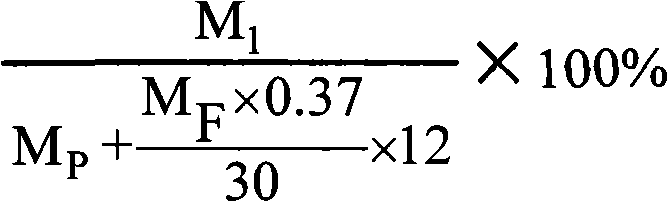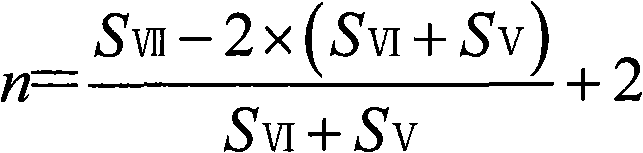Method for synthesizing o-cresol novolac resin
A technology of novolak resin and o-cresol, applied in the field of synthesizing o-cresol series novolak resin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example
[0068] The synthesis examples and comparative examples of the present invention are described below. It must be pointed out that there are many synthetic examples of the present invention that have application value and can be implemented, and only a part of representative examples are listed here.
Synthetic example 1
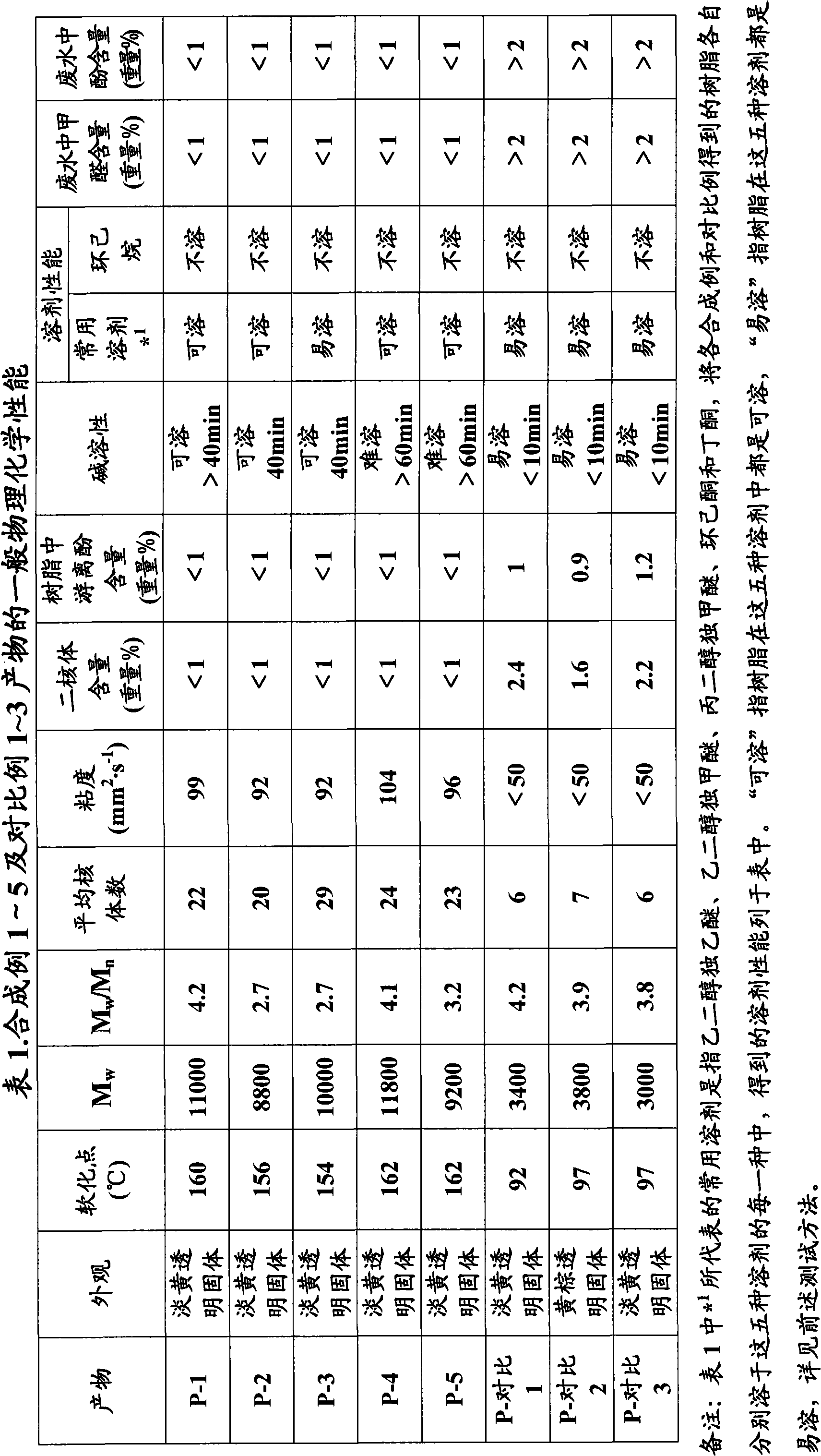
[0070]Get 216kg (2kmol) of o-cresol, 37% by weight of aqueous formaldehyde solution 162.2kg (2kmol) and 0.864kg of sodium hydroxide and join in the upper layer enamel reactor of double-layer reactor, and this upper layer enamel reactor adopts steam heating and is equipped with Vertical and horizontal condensers and water collection tanks. The temperature was raised to 70°C with stirring and maintained for 5 hours, and then maintained at 80°C, 90°C, and the reflux temperature of the reaction system for 1 hour, respectively. Then heat up the effluent, when the effluent reaches about 1 / 3 of the total effluent, cool down to 80~90 ℃, neutralize the reaction system to pH value 5 with concentrated hydrochloric acid solution, then add oxalic acid 2.16 % based on the total phenolic amount of 1% by weight kg, then the temperature was raised to the reflux temperature of the reaction system in about 1 hour, and the temperature was further increased to produce water, and then the temperatu...
Synthetic example 2
[0072] 162.2 kg of 37 wt % formaldehyde aqueous solution in Synthesis Example 1 was changed to 154.2 kg of 37 wt % formaldehyde aqueous solution (1.9 kmol), and the others were completely the same as Synthetic Example 1. The crude product P-2 was obtained in 98% yield. The general physicochemical properties of the resin as well as the formaldehyde and phenol content in the wastewater and the free phenol content in the resin are listed in Table 1, and the imaging properties are listed in Table 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Softening point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com