2-(1,2,4-triazolyl)benzoyl arylamine active compound for inhibiting pathogens causing take-all disease of wheat
A technology for wheat take-all disease and benzoyl arylamide, which is applied in the field of compounds for preventing and controlling crop diseases and insect pests, can solve the problems of limited control effect of wheat take-all disease and few varieties, and achieves excellent inhibitory effect, smooth synthesis process, and guaranteed production safety. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
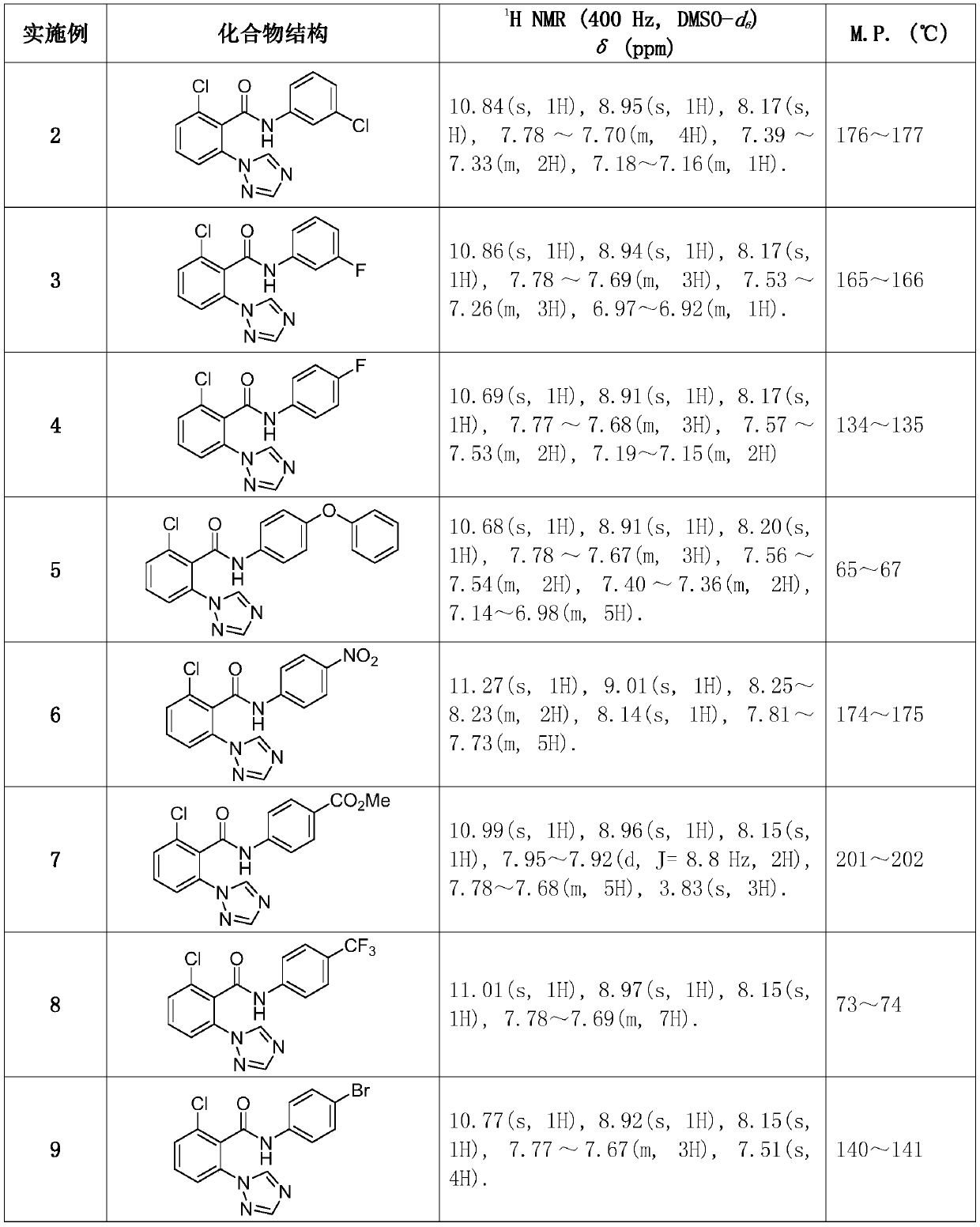
[0045] 2-Chloro-N-(4-chlorophenyl)-6-(1H-1, 2, 4-triazol-1-yl)benzamide
[0046] 2-chloro-N-(4-chlorophenyl)-6-(1H-1,2,4-triazol-1-yl)benzamide
[0047]
[0048]Step 1 Mix 0.17 g (1 mmol) 2, 6-dichlorobenzonitrile, 0.09 g (1.3 mmol) technical grade 1, 2, 4-triazole, 0.43 g (1.3 mmol) anhydrous cesium carbonate and 9.5 mg (0.05 mmol) CuI and 8.1 mg (0.05 mmol) 8-hydroxyquinoline-N-oxide ligand were added to a 20 mL small reaction vial, and then 3 mL of N, N-dimethylformamide was measured, Add it to the reaction flask, place it in an oil bath at 85 °C, stir and heat for 12 h. After the reaction was complete as detected by TLC, potassium carbonate and unreacted triazole were filtered under reduced pressure, the filtrate was washed with water, extracted, concentrated, and separated by thin-layer chromatography to obtain 133 mg of off-white solid. 2-Chloro-6-(1H-1, 2, 4-triazol-1-yl)benzonitrile, off-white solid, melting point: 170℃-172 ℃ Yield: 65%. 1 H NMR (400 MHz, CDCl 3...
Embodiment 2~23
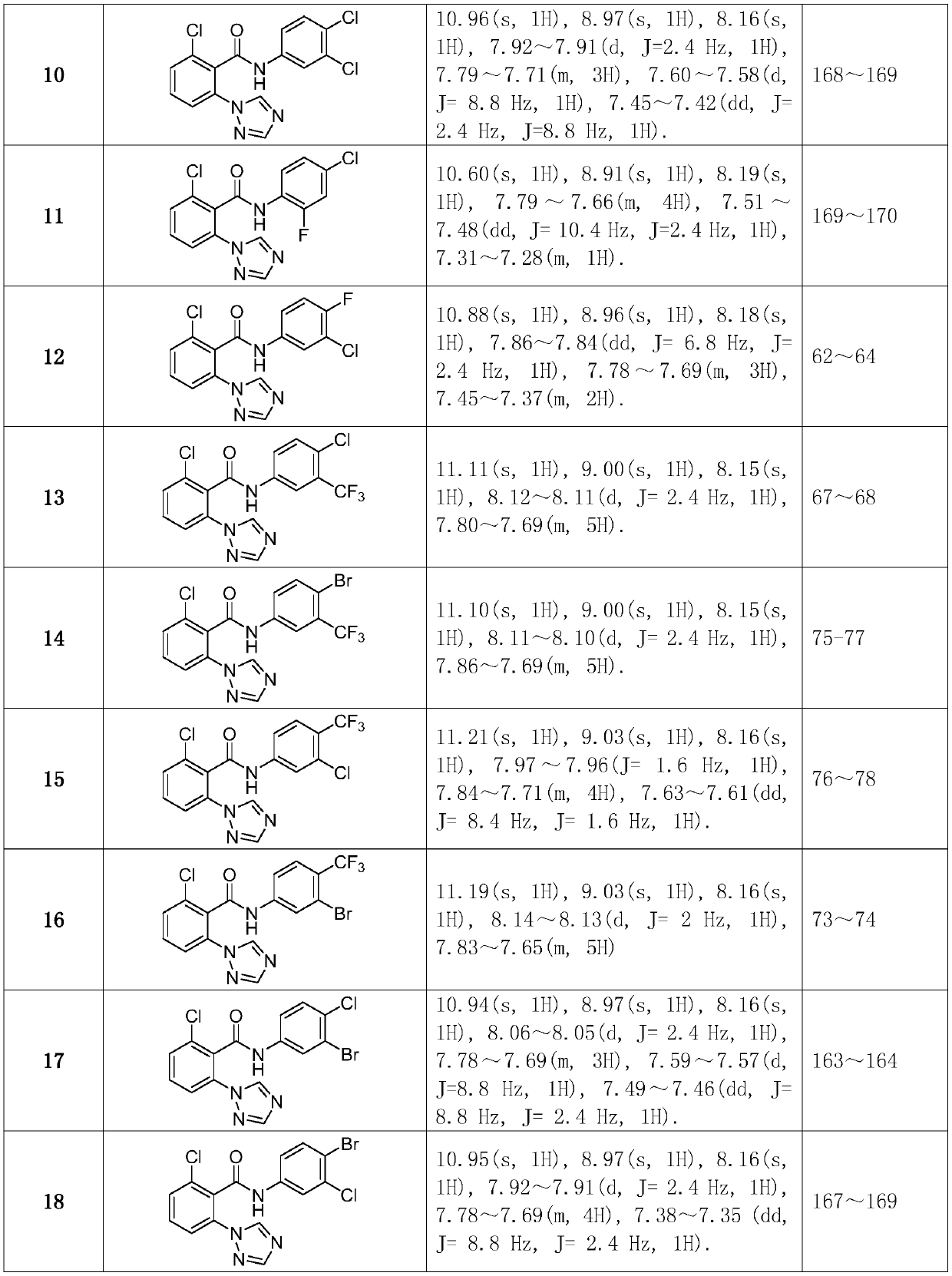
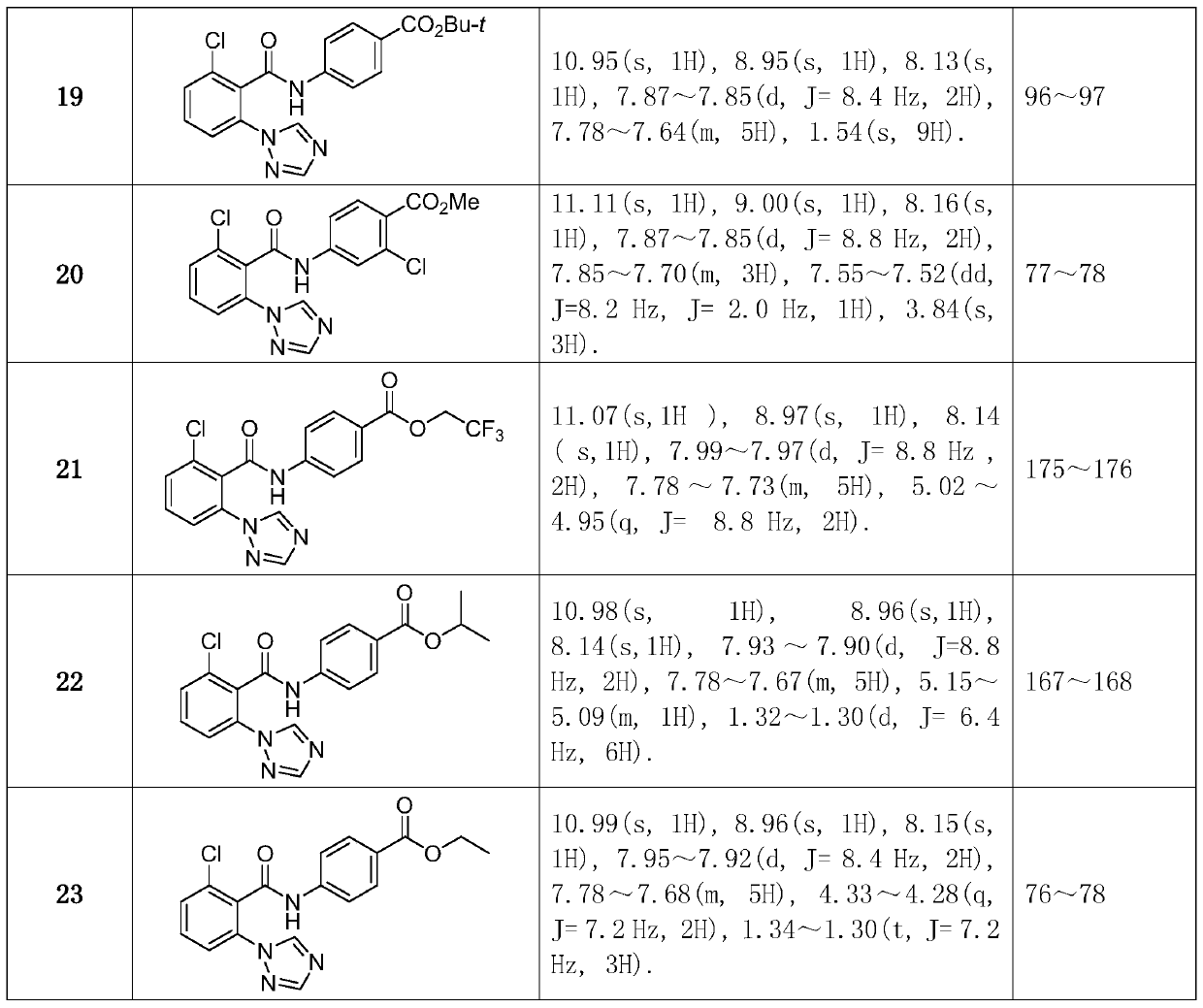
[0053] Synthesized according to the same method as in Example 1. The intermediate 2-chloro-6-(1H-1,2,4-triazol-1-yl)benzoic acid synthesized in Example 1 was used as a raw material, reacted with different substituted anilines, synthesized and separated. Compound structure, some physical and chemical parameters and characterization data such as figure 1 shown.
Embodiment 24~25
[0055] Using 2-chlorobenzonitrile and 2,3-dichlorobenzonitrile as raw materials respectively, the corresponding 2-(1H-1,2,4-triazol-1-yl) was synthesized according to the same method as in step 1 of Example 1 Benzonitrile and 3-chloro-2-(1H-1,2,4-triazol-1-yl)benzonitrile; ) benzonitrile and 3-chloro-2-(1H-1,2,4-triazol-1-yl) benzonitrile are intermediates, according to the same method as in Example 1 step 2,3, the substituted cyanide is hydrolyzed Obtain 2-(1H-1,2,4-triazol-1-yl) benzoic acid and 3-chloro-2-(1H-1,2,4-triazol-1-yl) benzoic acid respectively; In the same manner as in step 4 of Example 1, the obtained acid was condensed with isopropyl 4-aminobenzoate to obtain the target compound. Compound structure, some physical and chemical parameters and characterization data such as figure 2 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com