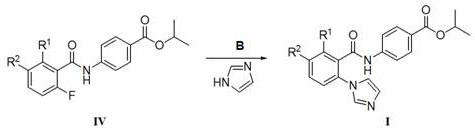
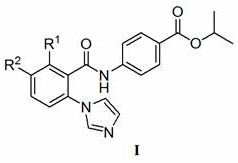
2-imidazole benzoyl arylamine active compound for preventing and treating wheat take-all and wheat stem rot
A technology of wheat take-all and benzoyl arylamine, which is applied in the field of compounds for preventing and controlling crop diseases and insect pests, can solve the problems that there are few varieties of fungicides for various root diseases of wheat, and achieve easy popularization and application, short synthesis steps, and easy raw materials The effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Synthesis of isopropyl 4-[(2-imidazol-1-yl)-6-fluorobenzamido]benzoate
[0030]
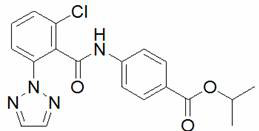
[0031] Step 1: Put 5.7 mmol of 2,6-difluorobenzoic acid into a three-necked reaction flask filled with 50 mL of dichloromethane and equipped with a reflux and gas absorption device, and slowly add 22.7 mmol of chlorinated chlorinated under normal temperature conditions Sulfone (SOCl 2 ) and 1ml DMF, heated to 40 o C continued to heat the reaction for 4 h. TLC detects that the reaction is complete, remove the solvent and excess thionyl chloride under negative pressure, then add 50 mL of fresh toluene to the reaction flask, and weigh 6.1 mmol 4-aminobenzoic acid isopropyl ester in batches to add to the reaction bottle, at 100 o Reaction 4 h under the temperature of ℃; TLC detects that after the reaction finishes, the reaction solution obtains the crude product of 4-(2,6-difluorobenzamido) isopropyl benzoate through neutralization, water washing and precipitation, and then passes throug...
Embodiment 2
[0034] Synthesis of isopropyl 4-[(2-imidazol-1-yl)-6-chlorobenzamido]benzoate
[0035]
[0036] Step 1: Put 5 mmol of 2-fluoro-6-chlorobenzoic acid into a three-necked reaction flask filled with 50 mL of dichloromethane and equipped with a reflux and gas absorption device, and slowly add 6 mmol of oxalyl chloride at room temperature (COCl) 2 and a few drops of DMF and incubated for 4 h. TLC detection until the reaction is complete, remove the solvent and excess oxalyl chloride under negative pressure, then add 50 mL of fresh toluene to the reaction flask, and weigh 10 mmol 4-aminobenzoic acid isopropyl ester in batches , at 100 o Reaction 4 h under ℃ temperature; After TLC detects that reaction finishes, reaction solution obtains the crude product of 4-(2-fluoro-6-chlorobenzamido) isopropyl benzoate through neutralization, water washing, stripping, then passes column Chromatographic separation gives a white solid;
[0037] Step 2: Mix 2 mmol 4-(2-fluoro-6-chlorobenzamid...
Embodiment 3
[0039] Synthesis of isopropyl 4-[(2-imidazol-1-yl)-6-bromobenzamido]benzoate
[0040]
[0041] Step 1: Put 5 mmol of 2-fluoro-6-bromobenzoic acid into a three-necked reaction flask filled with 50 mL of dichloromethane and equipped with a reflux and gas absorption device, and slowly add 6 mmol of chlorinated Sulfoxide SOCl 2 and a few drops of DMF, and incubated for 6 h. TLC detects that until the reaction is complete, remove the solvent and excess thionyl chloride under negative pressure, then add 50 mL of fresh toluene to the reaction flask, and weigh 7.5 mmol of isopropyl 4-aminobenzoate to add to the reaction in batches. bottle, at 100 o Reaction at temperature of C for 6 h; after TLC detection of the reaction, the reaction solution was neutralized, washed with water, and stripped to obtain the crude product of 4-(2-fluoro-6-bromobenzamido) isopropyl benzoate, and then passed through the column Chromatography afforded a white solid.
[0042] Step 2: Mix 2 mmol 4-(2-f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com