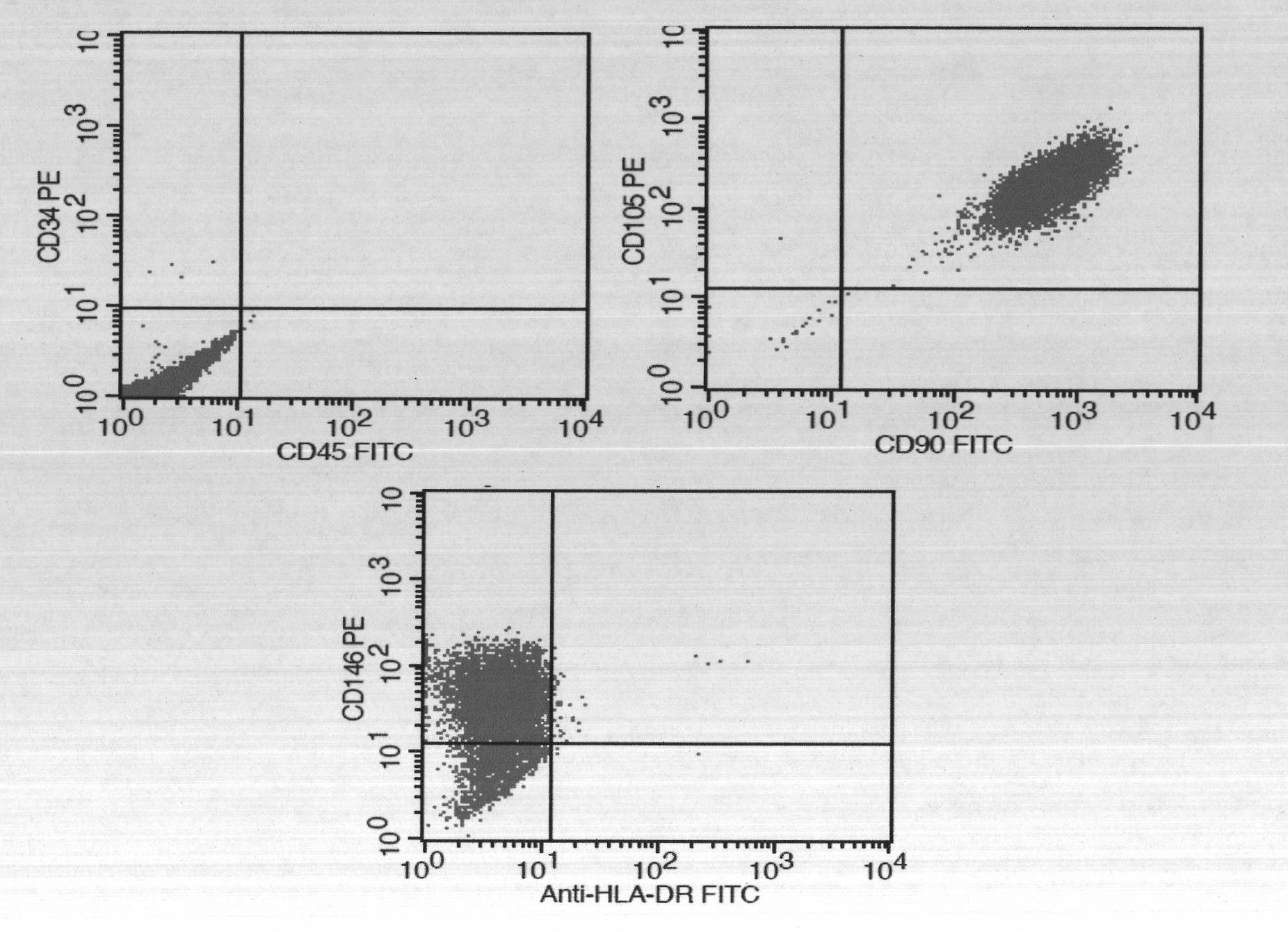
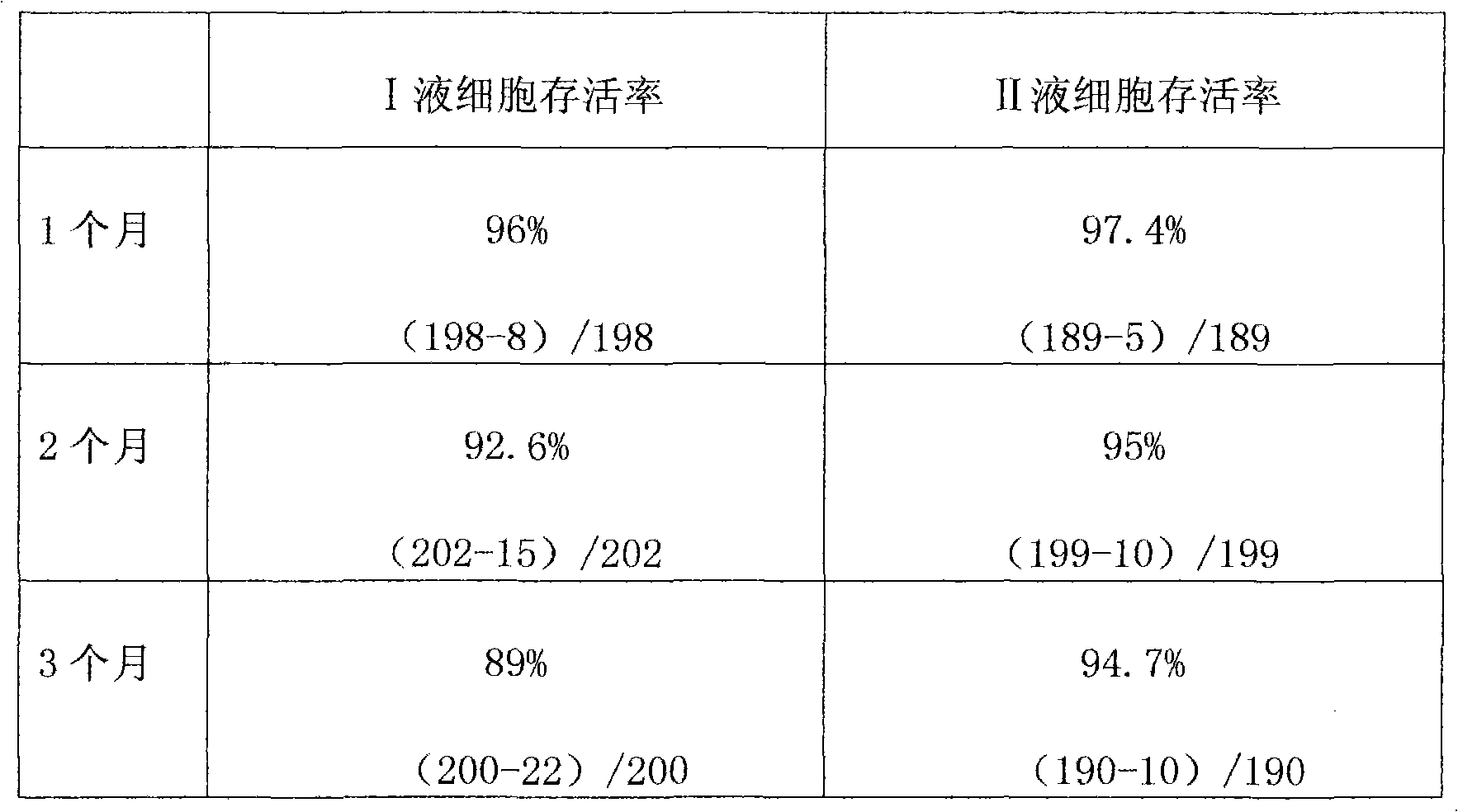
Mesenchymal stem cell cryopreserving liquid directly applied to veins
A technique for mesenchymal stem cells and cryopreservation solution, applied in the field of mesenchymal stem cell cryopreservation solution and its preparation, can solve the problems of potential safety hazards, high price, differences in cell preparation conditions and characteristics, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0023] 1. Preparation of human AB (type) plasma for cryopreservation
[0024] (1) Human AB blood (purchased from Qingdao Central Blood Center) containing 2.5% sodium citrate by mass (may not be included, does not affect the technical effect of the present invention) is placed in a sterile centrifuge tube with a capacity of 50 ml, Centrifuge at 1000rpm for 15 minutes;
[0025] (2) Aspirate the supernatant, transfer it into a new centrifuge tube, and centrifuge at 4000g for 20 minutes to remove platelets. The supernatant is human AB plasma for cryopreservation, which is used in the following 2 steps.
[0026] 2. Preparation of mesenchymal stem cell cryopreservation medium
[0027] Solution I: DMSO (dimethyl sulfoxide, purchased from SIGMA), human AB plasma for cryopreservation, and Bomaili A injection (purchased from Shanghai Baite) were mixed uniformly at a volume ratio of 1:3:6, and placed at 4°C spare;
[0028] Solution II: DMSO, fetal bovine serum (purchased from GIBCO), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com