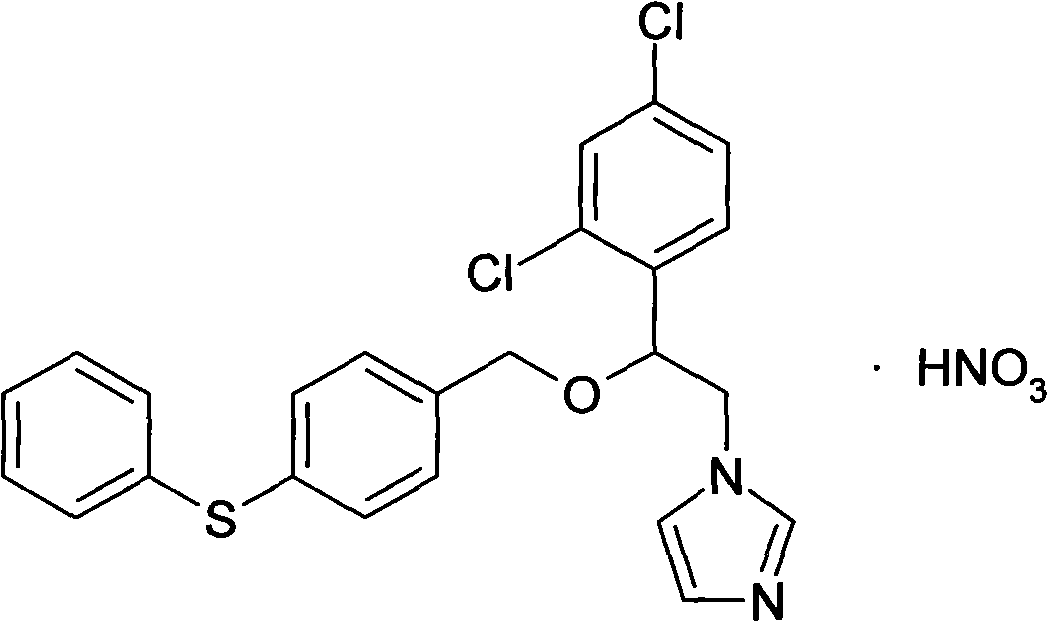
Fenticonazole nitrate vaginal suppository composition
A technology for fenticonazole nitrate and teconazole suppositories, applied in the directions of suppository delivery, drug combination, antifungal agent, etc., can solve problems such as high cost, complicated process operation, insufficient production line, etc., and achieves repair of diseased tissue and mature technology. , to avoid the effect of gastric acid damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The prescription consists of:
[0025] (a) Fenticonazole Nitrate 200mg / capsule
[0026] (b) Semi-synthetic fatty acid glyceride (type 36) 2300mg / capsule
[0027] Taking the preparation of 1000 fenticonazole nitrate vaginal suppositories as an example, the specific preparation method is to grind and pulverize fenticonazole nitrate with a rotary mill or an air jet ultra-fine grinder. The particle size of the drug particles is not greater than 30 μm. Heat and melt the semi-synthetic fatty acid glyceride (type 36) in the preparation tank to 50°C ± 5°C, maintain the temperature of the melting matrix at 50°C ± 5°C, add the prescribed amount of fenticonazole nitrate into the above preparation tank , mix it well and homogenize to obtain a homogeneous dispersion. Maintaining mixing and maintaining the temperature at 45°C ± 5°C, the drug dispersion is formed into suppositories using an automatic form / fill / seal suppository machine.
Embodiment 2
[0029] The prescription consists of:
[0030] (a) Fenticonazole Nitrate 200mg / capsule
[0031] (b) Semi-synthetic fatty acid glyceride (type 38) 2300mg / capsule
[0032] Taking the preparation of 1000 fenticonazole nitrate vaginal suppositories as an example, the specific preparation method is to grind and pulverize fenticonazole nitrate with a rotary mill or an air jet ultra-fine grinder. The particle size of the drug particles is not greater than 30 μm. Heat and melt the semi-synthetic fatty acid glyceride (type 38) to 50°C±5°C in the preparation tank, and melt the base
[0033] The temperature of the substance was maintained at 50°C ± 5°C, and the prescribed amount of fenticonazole nitrate was added into the above preparation tank, mixed and homogenized to obtain a uniform dispersion. Maintaining mixing and maintaining the temperature at 45°C ± 5°C, the drug dispersion is formed into suppositories using an automatic form / fill / seal suppository machine.
Embodiment 3
[0035] The prescription consists of:
[0036] (a) Fenticonazole Nitrate 200mg / capsule
[0037] (b) Semi-synthetic fatty acid glyceride (type 36) 1800mg / capsule
[0038] Taking the preparation of 1000 fenticonazole nitrate vaginal suppositories as an example, the specific preparation method is to grind and pulverize fenticonazole nitrate with a rotary mill or an air jet ultra-fine grinder. The particle size of the drug particles is not greater than 30 μm. Heat and melt the semi-synthetic fatty acid glyceride (type 36) in the preparation tank to 50°C ± 5°C, maintain the temperature of the melting matrix at 50°C ± 5°C, add the prescribed amount of fenticonazole nitrate into the above preparation tank , mix it well and homogenize to obtain a homogeneous dispersion. Maintaining mixing and maintaining the temperature at 45°C ± 5°C, the drug dispersion is formed into suppositories using an automatic form / fill / seal suppository machine.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com