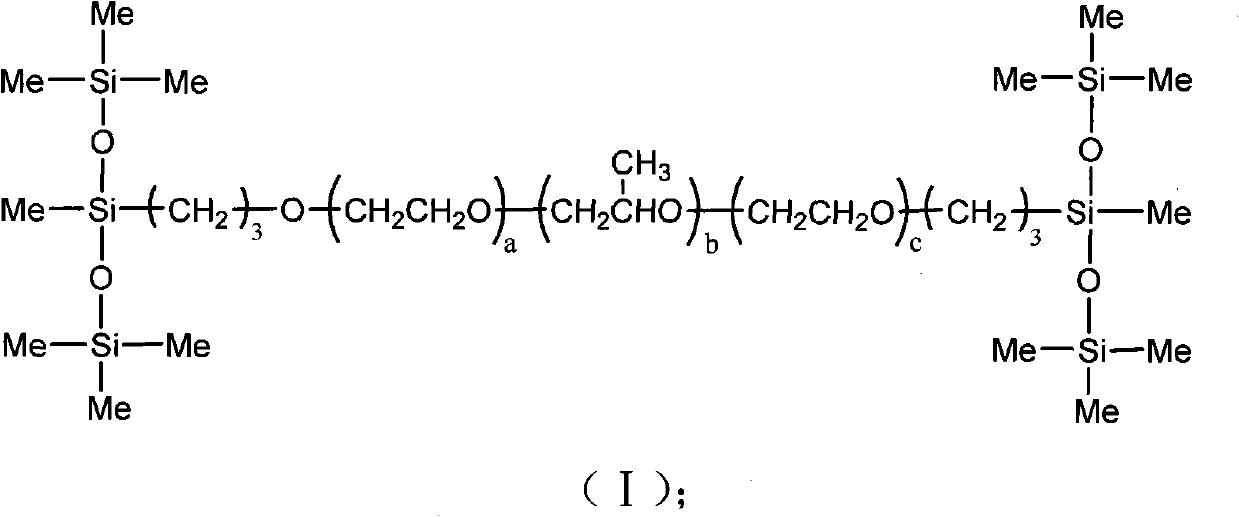Organic silicon compounds, and preparation method and application thereof
A technology of organosilicon compounds and hydroxides, applied in the direction of silicon organic compounds, organic chemistry, chemical instruments and methods, etc., can solve the problems of limited effect of oil collectors and inconvenient use, and achieve low surface tension, convenient use, and concentrated good oil effect
Active Publication Date: 2011-01-12
GUANGDONG BIOMAX SIANDF NEW MATERIAL CO LTD
View PDF8 Cites 11 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
The purpose of the present invention is to solve the problems of limited effect and inconvenient use of the oil-collecting agent in the prior art, and provide a novel organosilicon compound, which has a unique structure and is effective in driving various hydrocarbon oil spills. It has good effect, is easy to use, and is environmentally friendly, and can be used as an oil collector for oil spill treatment in waters
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
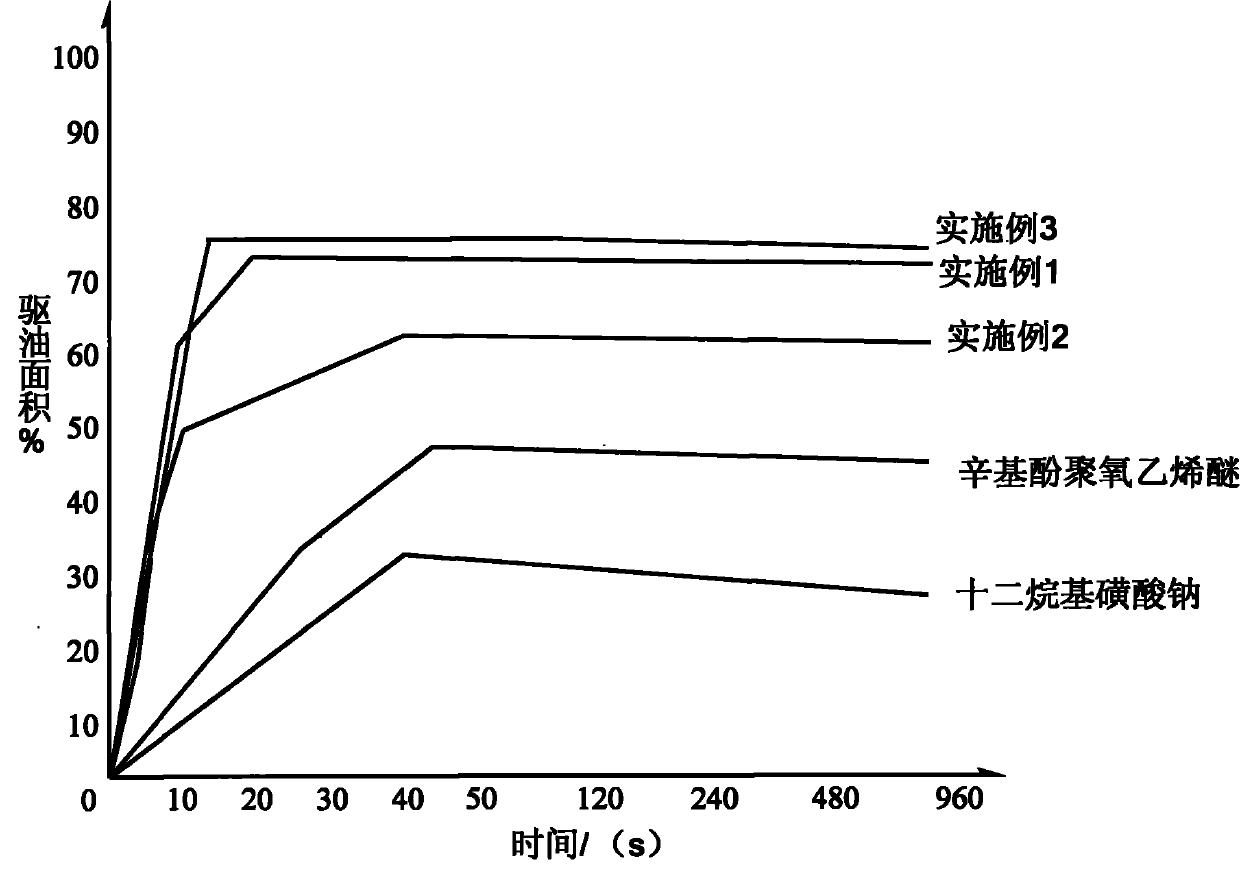
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
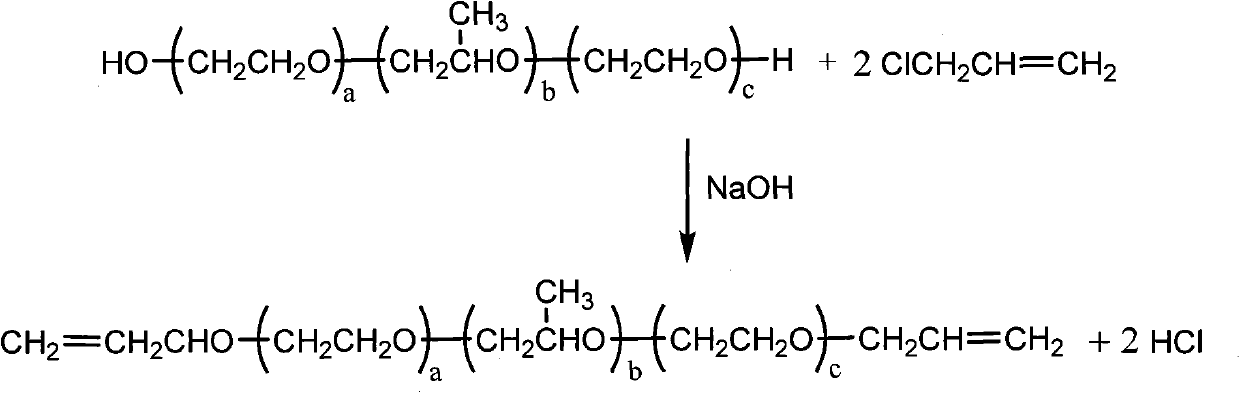
The invention discloses organic silicon compounds. The organic silicon compounds have the structure shown in a formula (I), wherein a is equal to 0 to 40, b is equal to 0 to 30, c is equal to 0 to 40, a, b and c are all integers and the sum of a, b and c is more than 6. the invention discloses a method for preparing the organic silicon compounds, and the method comprises the following steps of: reacting alpha-hydrogen-omega-hydroxyl polyether with halogenated propylene which serve as raw materials in the presence of alkali metal hydroxides to form diallyl polyether; and reacting the obtained diallyl polyether with 1,1,1,3,5,5,5-heptamethyltrisiloxane in the presence of a Pt catalyst to obtain the finished product. The organic silicon compounds have the advantages of unique structure, low surface tension, no toxin and high oil-collecting effect, and can be widely applied to the field of oil spill pollution treatment in water areas.
Description
technical field The invention belongs to the technical field of organosilicon compound synthesis, and in particular relates to an organosilicon compound and its preparation method and application. Background technique Silicone surfactant is a new type of surfactant developed along with new silicone materials. Like fluorine-containing surfactants, it has won a broad space for survival and development with its unique properties since it came out. Now it has become an increasingly important special surfactant. Offshore oil exploration and transportation often cause oil spills and oil spills due to accidents or other technical reasons, which not only cause huge economic losses to enterprises, but also cause serious environmental pollution. After the oil spills in the water, it will spread out quickly and form a very thin oil layer, making recovery difficult. The oil collector based on surfactant can reduce the surface tension of seawater, reduce the area of oil spill and fo...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C08G65/00C07F7/08C02F1/40
CPCC02F2101/32C08G77/485C07F7/0852C08G65/336C02F1/681C02F1/682C08G77/46C07F7/0838
Inventor 黄振宏谢秀鸿黄晓梅
Owner GUANGDONG BIOMAX SIANDF NEW MATERIAL CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com