Novel heterocycles
A general formula and compound technology, which can be used in medical preparations containing active ingredients, allergic diseases, metabolic diseases, etc., and can solve problems such as side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
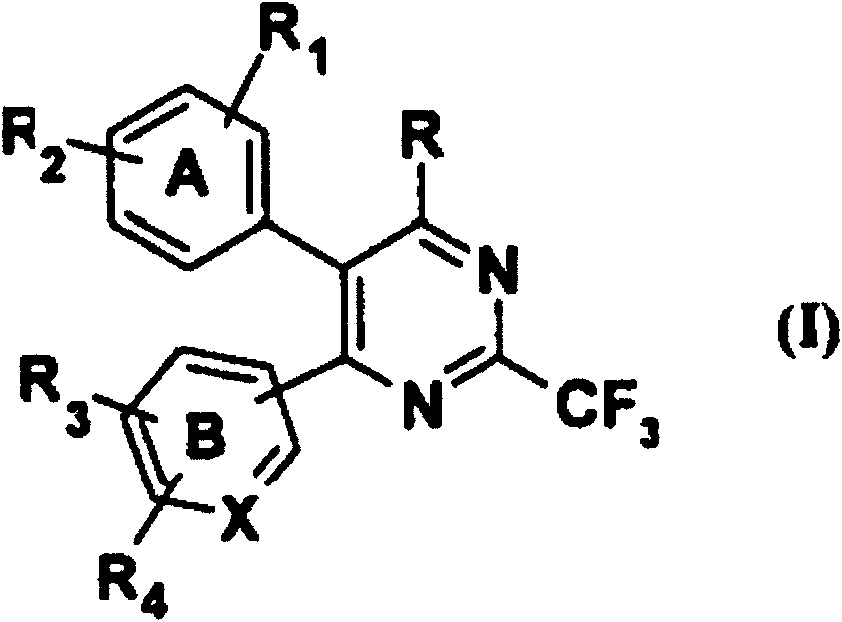
[0271] According to another embodiment of the present invention, there is provided a method for preparing a novel heterocyclic compound of formula (I),
[0272]
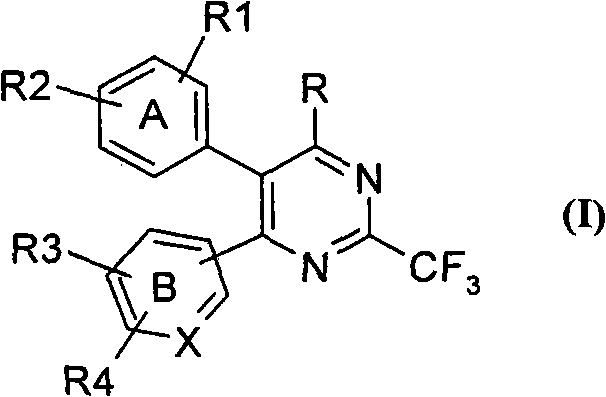
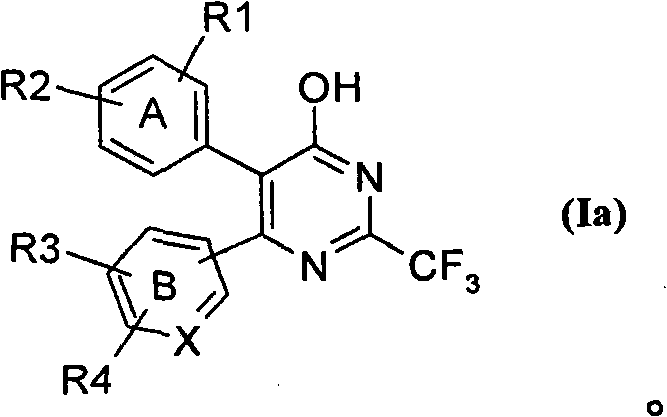
[0273] wherein B represents pyridine and R represents a halogen atom, which can be prepared by transforming compounds of formula (Ia), wherein all symbols are as defined above.
[0274]
[0275] The compound of formula (Ia) was prepared following the procedures described in our PCT / IB03 / 01289.
[0276] with or without the presence of solvents such as toluene, xylene, tetrahydrofuran, di alkanes, chloroform, dichloromethane, dichloroethane, o-dichlorobenzene, diphenyl ether, etc., or mixtures thereof, with or without catalytic amounts of dimethylformamide or N,N-dimethylaniline or N, Compounds of formula (Ia) are converted using halogenating agents such as phosphorus oxychloride, thionyl chloride, phosphorus trichloride, phosphorus pentachloride and the like in the presence of N-diethylaniline and the like. T...
preparation example 1
[0334] Synthesis of 6-[4-(methylsulfonyl)phenyl]-5-phenyl-2-(trifluoromethyl)pyrimidin-4-amine
[0335]
[0336] At 0-10°C, 4-chloro-6-[4-(methylsulfonyl)phenyl]-5-phenyl-2-(trifluoromethyl)pyrimidine (5.5g, 13.33mmol, according to PCT / IB03 / 02879 described process preparation) THF (500ml) solution is fed continuously into ammonia gas and reaches 30 hours, stirs simultaneously. After completion of the reaction, THF was completely distilled in vacuo, water (100ml) was added and the reaction mixture was extracted with ethyl acetate (3x200ml). The combined organic layers were washed with brine (150ml), dried over anhydrous sodium sulfate, and concentrated under reduced pressure to give 6-[4-(methylsulfonyl)phenyl]-5-phenyl-2-(trifluoromethyl ) pyrimidin-4-amine (3.6 g, yield 87.79%).
[0337] Prepare the following compounds according to the above process
[0338]
Embodiment 3
[0340] Synthesis of 4-{4-chloro-6-[4-(methylsulfonyl)phenyl]-2-(trifluoromethyl)pyrimidin-5-yl}benzenesulfonyl chloride
[0341]
[0342] To chlorosulfonic acid solution (605mmol, 40.4ml) was slowly added 4-chloro-6-[4-(methylsulfonyl)phenyl]-5-phenyl-2-(trifluoromethyl)pyrimidine (5.0g, 12.1 mmol, prepared according to the procedure described in PCT / IB03 / 02879), stirring was continued at 0°C until the addition was complete. The reaction mixture was then continued to stir at 32 °C until TLC confirmed the completion of the reaction. The resulting reaction was then slowly poured onto crushed ice under vigorous stirring, and the resulting solid was filtered, washed well with water (100ml), and extracted with ethyl acetate (3x200ml). The combined organic layers were washed with brine (150ml), dried over anhydrous sodium sulfate, and concentrated under reduced pressure to give 4-{4-chloro-6-[4-(methylsulfonyl)phenyl]-2-(trifluoro Methyl)pyrimidin-5-yl}benzenesulfonyl chloride ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com