Thiazoleamide compound and medical application thereof in treating malignant tumor
A technology of thiazole amides and compounds, which is applied in the field of preparation of anti-cancer drugs, can solve the problems of affecting bioavailability, low water solubility, large dosage, etc., and achieve effective bioavailability, high bioavailability, and high water solubility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
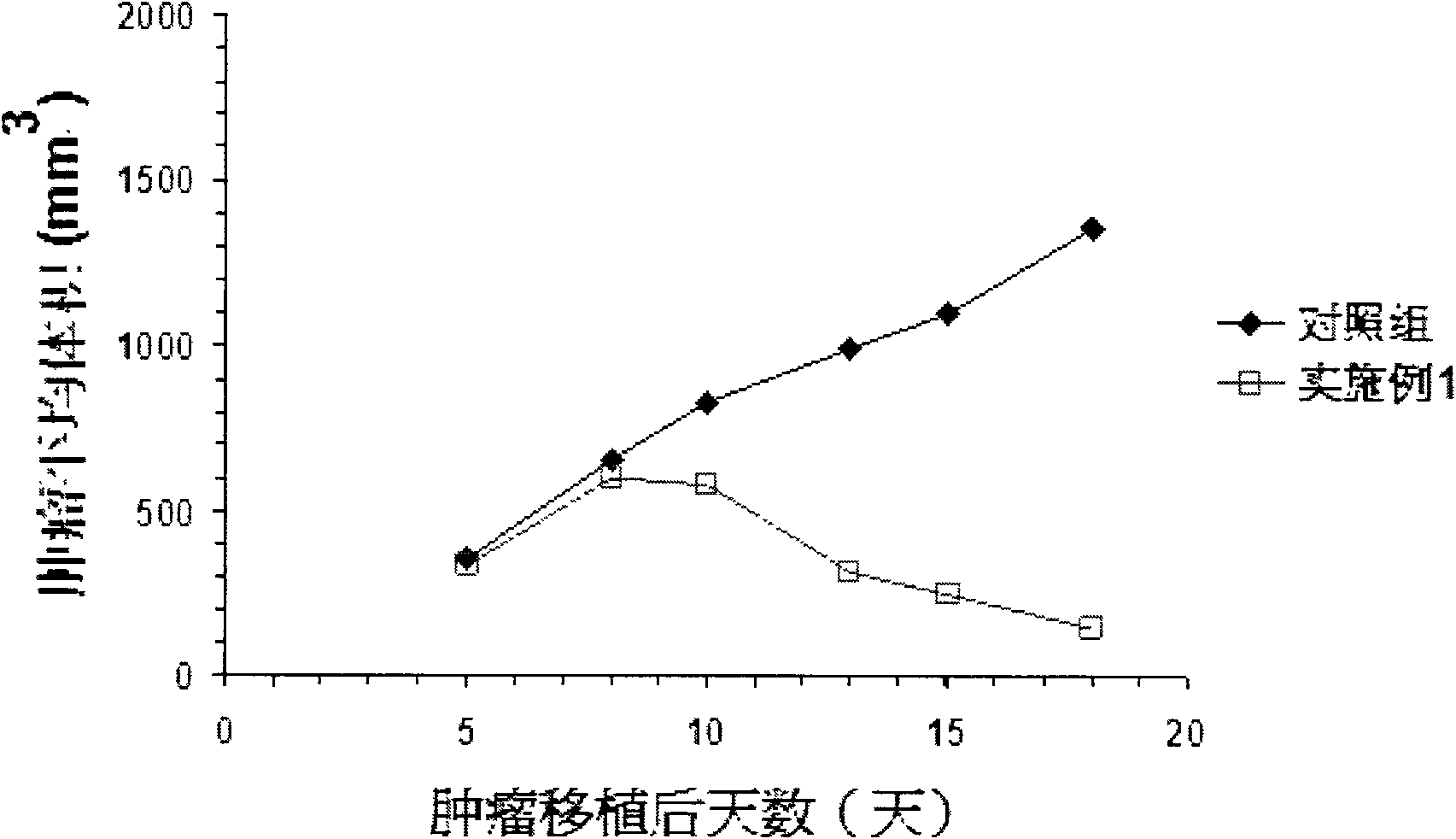
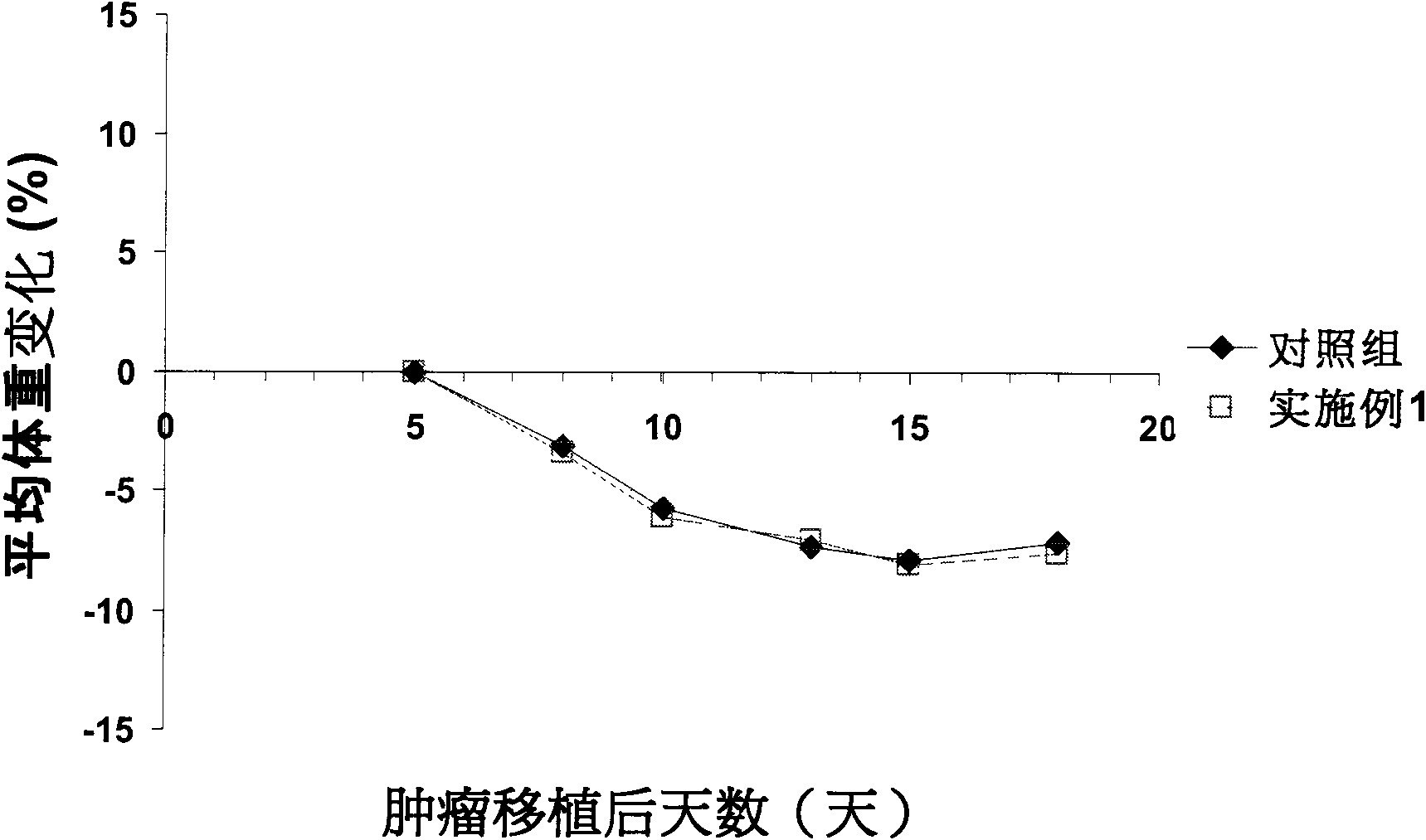
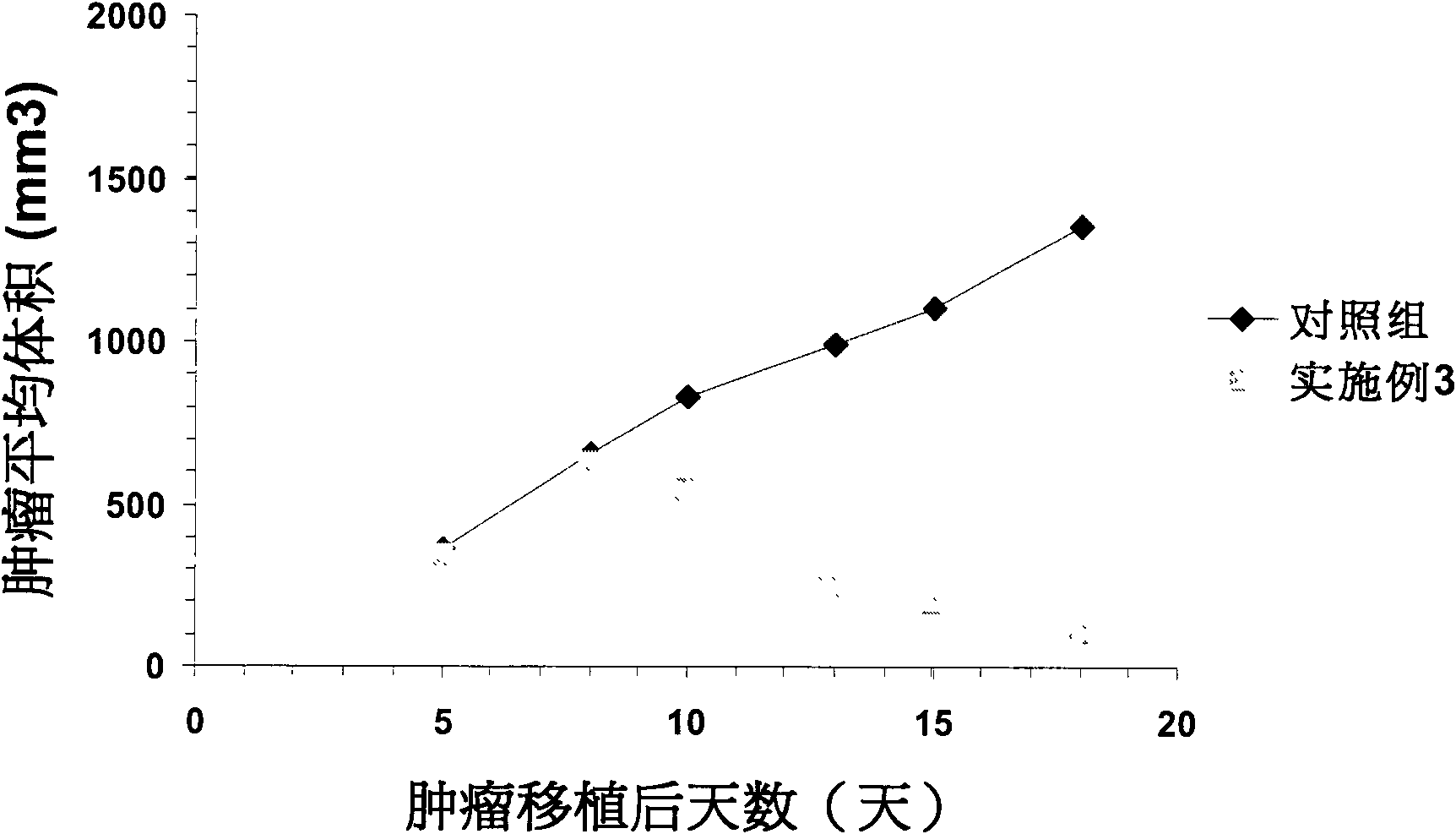
Image
Examples
Embodiment 1
[0040] N-(2-chloro-6-methylphenyl)-2-[[6-[4-tert-butoxycarbonyl-1-piperazinyl]-2-methyl-4-pyrimidinyl]amino]-5 -thiazolecarboxamide, its structural formula is as follows:
[0041]
[0042] Above-mentioned compound can obtain by following synthetic route:
[0043]
[0044] The specific preparation process is as follows: while stirring, 1-tert-butoxycarbonyl-piperazine (2.83g, 15.19mmol) and N, N-diisopropylethylamine (3.77g, 32.8mmol) were slowly added dropwise to N- (2-Chloro-6-methyl-phenyl)-2-[(6-chloro-2-methyl-4-pyrimidinyl)amino]-5thiazolecarboxamide (5g, 12.68mmol) In 100mL sulfone solution, heat to 115°C, react under stirring for 18 hours, cool to room temperature, add water, precipitate out, stir at room temperature for 15 minutes, filter, wash with ethyl acetate to obtain a light brown powder, and then use ethyl acetate The ester was recrystallized to obtain 5.4 g of pure beige product (78.3% yield).
[0045] The obtained taupe product was subjected to elemen...
Embodiment 2
[0050] N-(2-chloro-6-methylphenyl)-2-[[6-[4-ethoxycarbonyl-1-piperazinyl]-2-methyl-4-pyrimidinyl]amino]-5- Thiazole carboxamide, the structural formula is as follows:
[0051]
[0052] The compound can be prepared by making N-(2-chloro-6-methyl-phenyl)-2-[(6-chloro-2-methyl-4-pyrimidinyl)amino]-5thiazolecarboxamide with 1-ethyl Oxycarbonyl-piperazine is obtained through a substitution reaction, and the specific preparation process can be referred to Example 1.
Embodiment 3
[0054] N-(2-chloro-6-methylphenyl)-2-[[6-[4-tert-butylcarbonyl-1-piperazinyl]-2-methyl-4-pyrimidinyl]amino]-5- Thiazole carboxamide, the structural formula is as follows:
[0055]
[0056] The compound can be obtained by making N-(2-chloro-6-methyl-phenyl)-2-[(6-chloro-2-methyl-4-pyrimidinyl)amino]-5thiazolecarboxamide with 1-tert Butylcarbonyl-piperazine is obtained through a substitution reaction, and the specific preparation process can be found in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com