Monoclonal antibodies for tumor treatment
A monoclonal antibody, tumor technology, applied in the fields of therapy, antibodies, anti-tumor drugs, etc., can solve the problem of reducing anti-tumor activity and efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0179] Example 1. In vitro functional assays
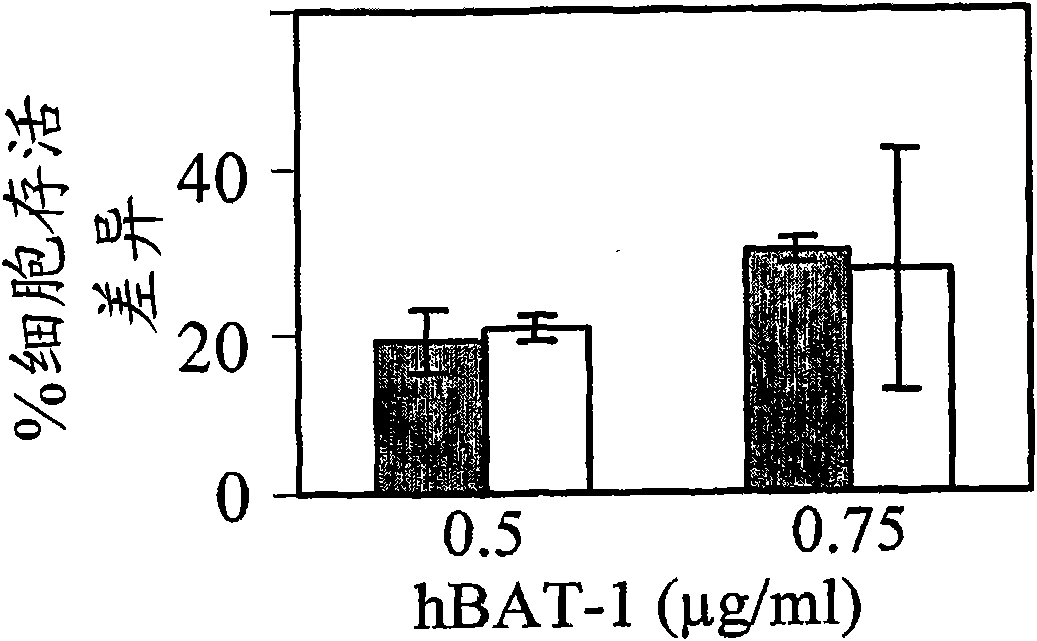
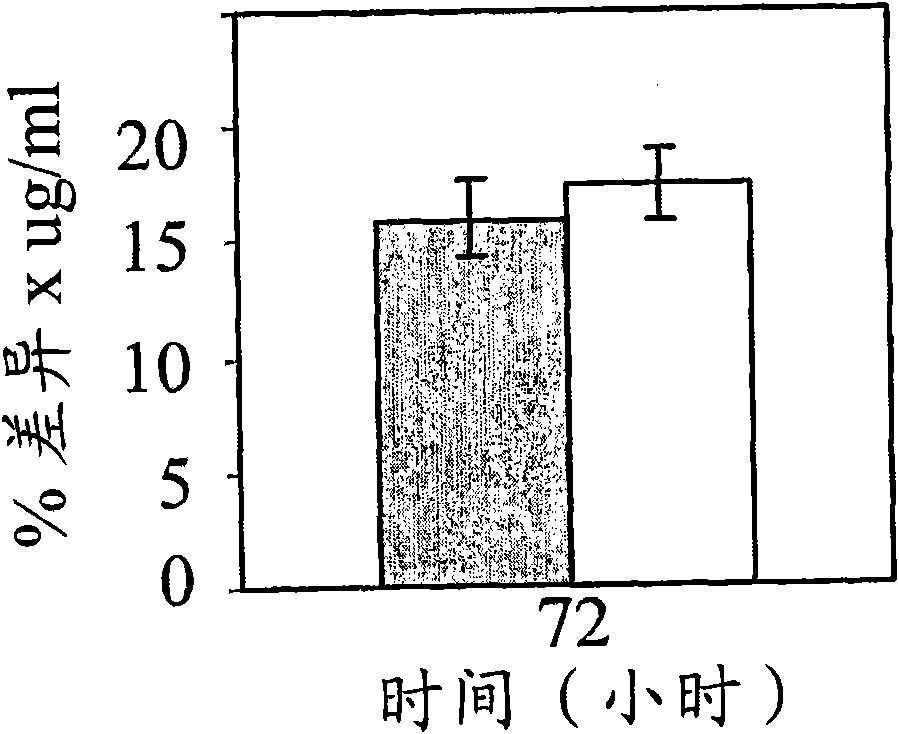
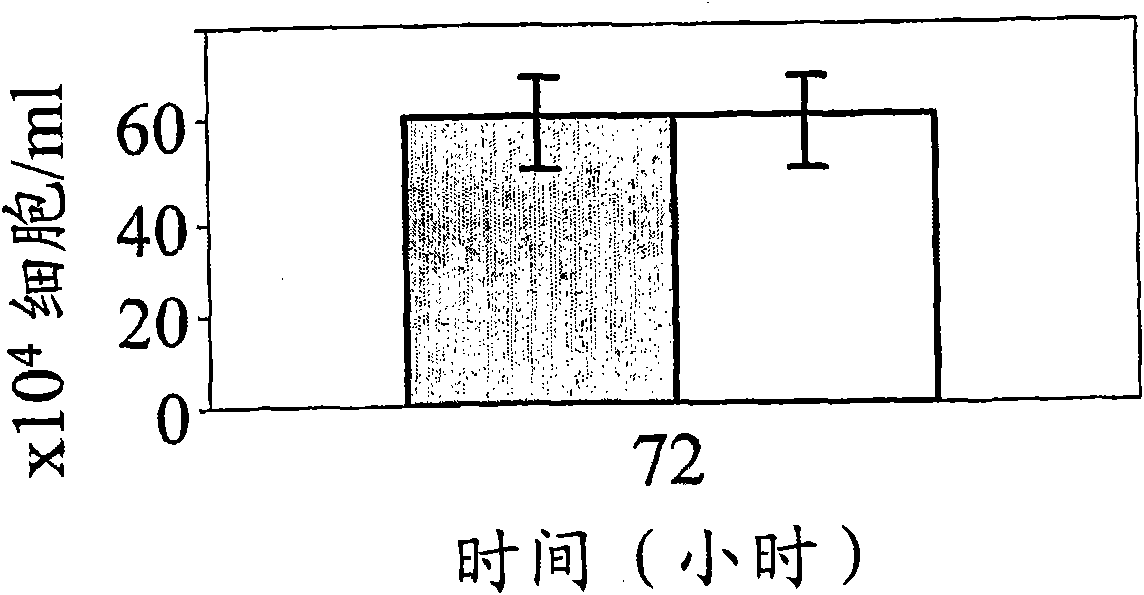
[0180] Functional assays are based on the ability of hBAT-1 to increase the survival of cultured murine and human lymphocytes. In this example, the effect of hBAT-1 alone or in combination with chemotherapeutic agents on the enhanced survival of lymphocytes was assessed and expressed by % difference in cell survival or by the area under the dose response curve (AUC expressed as % difference X μg / ml). Chemotherapeutic agents were administered with or 24 hours after hBAT-1 treatment at the indicated concentrations. Chemotherapeutic agents tested by functional assays included 5FU (Figures 1, 2, and 7), SN-38, active derivatives of irinotecan (Figures 3 and 4), cisplatin, oxaliplatin, Taxol (paclitaxel), and dacarba zine (Figures 5 and 7), cytarabine, cyclophosphamide, and doxorubicin (Figure 6).
[0181] The results indicate that specific agents (eg, 5FU, cisplatin, oxaliplatin, paclitaxel, and cytarabine) do not adversely affect...
Embodiment 2
[0182] Example 2. Combination therapy for colorectal cancer
[0183] Colorectal cancer (CT26 tumors) was induced by S.C. injection of CT26 cells, 10 6 Cells / mouse (n=6). The day of injection refers to Day 0. 20 mg / kg of 5-FU was administered I.P. on days 6-9, 15-17, 22-24 and 29-31 , 36-38 and 43-45. On days 10, 18, 25, 32 and 39, 10 mg / mouse of hBAT-1 ( Figure 8-10 ). Relapse cases after complete remission (observed only in the combination therapy group) were further administered with 20 mg / kg of 5FU on days 73-74, 77-80, 85-87, 92-93 and 10 mg / kg I.V. on days 81 and 88. hBAT-1 treatment of mice.
[0184] In a follow-up study of tumor size after one treatment cycle, tumor volumes were measured on alternate days from days 4 to 16 after tumor inoculation. The results showed that combined treatment with 5FU was superior to treatment with 5FU or hBAT-1 alone ( Figure 8 ).
[0185] In a follow-up study of tumor size after 3 alternating treatment cycles, tumor volumes w...
Embodiment 3
[0187] Example 3. Combination Therapy for Melanoma
[0188] with B16 melanoma cells at 5x10 5 Cells / mouse Mice (n=7) were inoculated subcutaneously. The day of inoculation refers to day 0. 50 mg / kg of 5-FU was administered intraperitoneally on days 1-4 and 7-8. In the combined treatment group, a single dose of 10 mg / mouse of hBAT-1 was injected intravenously on the 10th day.
[0189] Monitoring of percent survival began on day 8. The percent survival in mice treated with combination therapy was significantly higher than in mice treated with high doses of 5FU ( Figure 11 ).
[0190] Otherwise stated, combination therapy using a sequential administration schedule in which the humanized antibody was administered after a 9-day cycle of 5FU at dose-limiting toxicity (DLT) levels (50 mg / kg / day) resulted in enhanced survival of mice. The results clearly show that the combination therapy improves tolerance to DLT levels of 5-FU.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com