Immunologic suppression effect of hydroxycamptothecine and therapeutic effect of hydroxycamptothecine on rheumatoid arthritis
A hydroxycamptothecin, immunosuppressive technology, used in anti-inflammatory agents, bone diseases, non-central analgesics, etc., can solve problems such as adverse reactions in patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Embodiment 1: Hydroxycamptothecin treats CIA rats
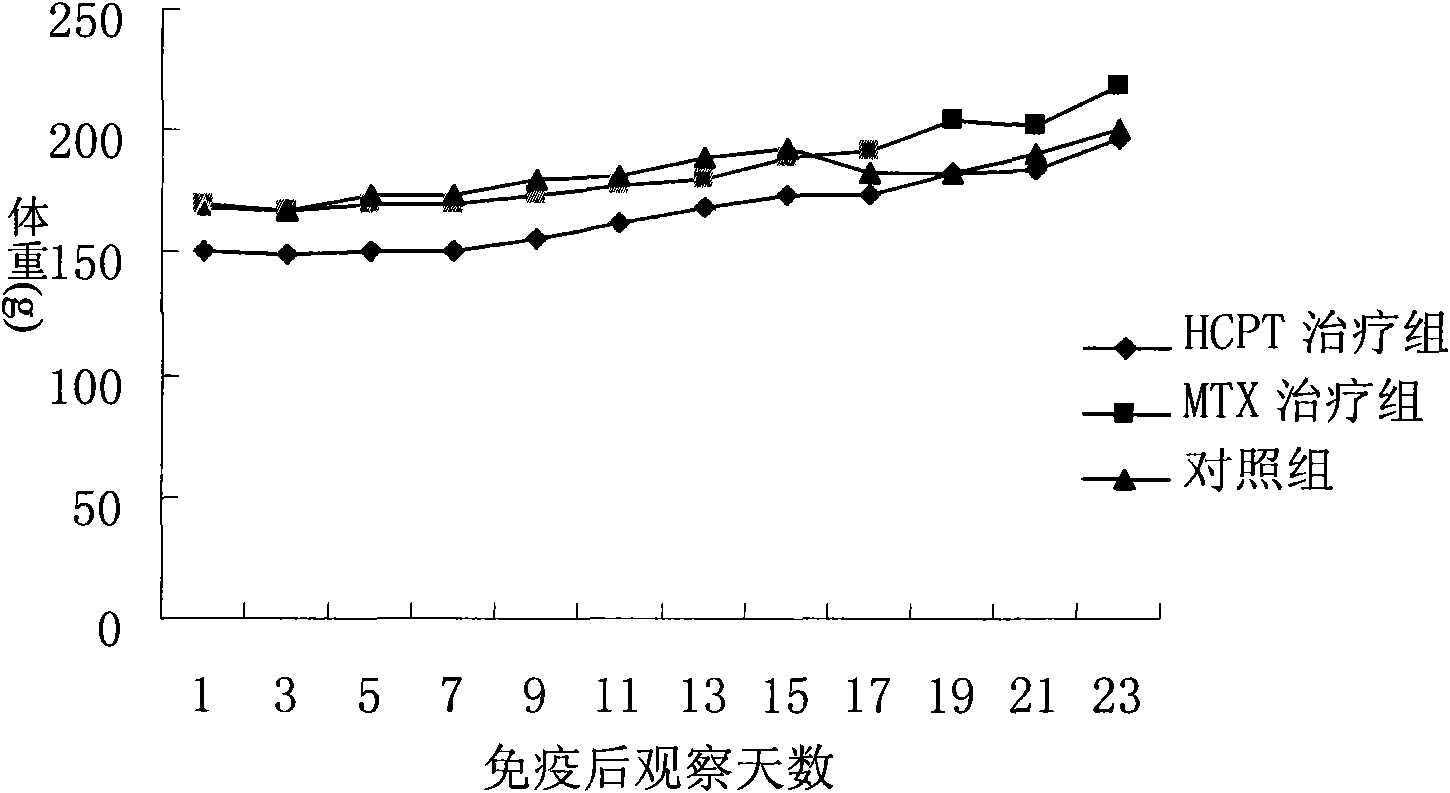
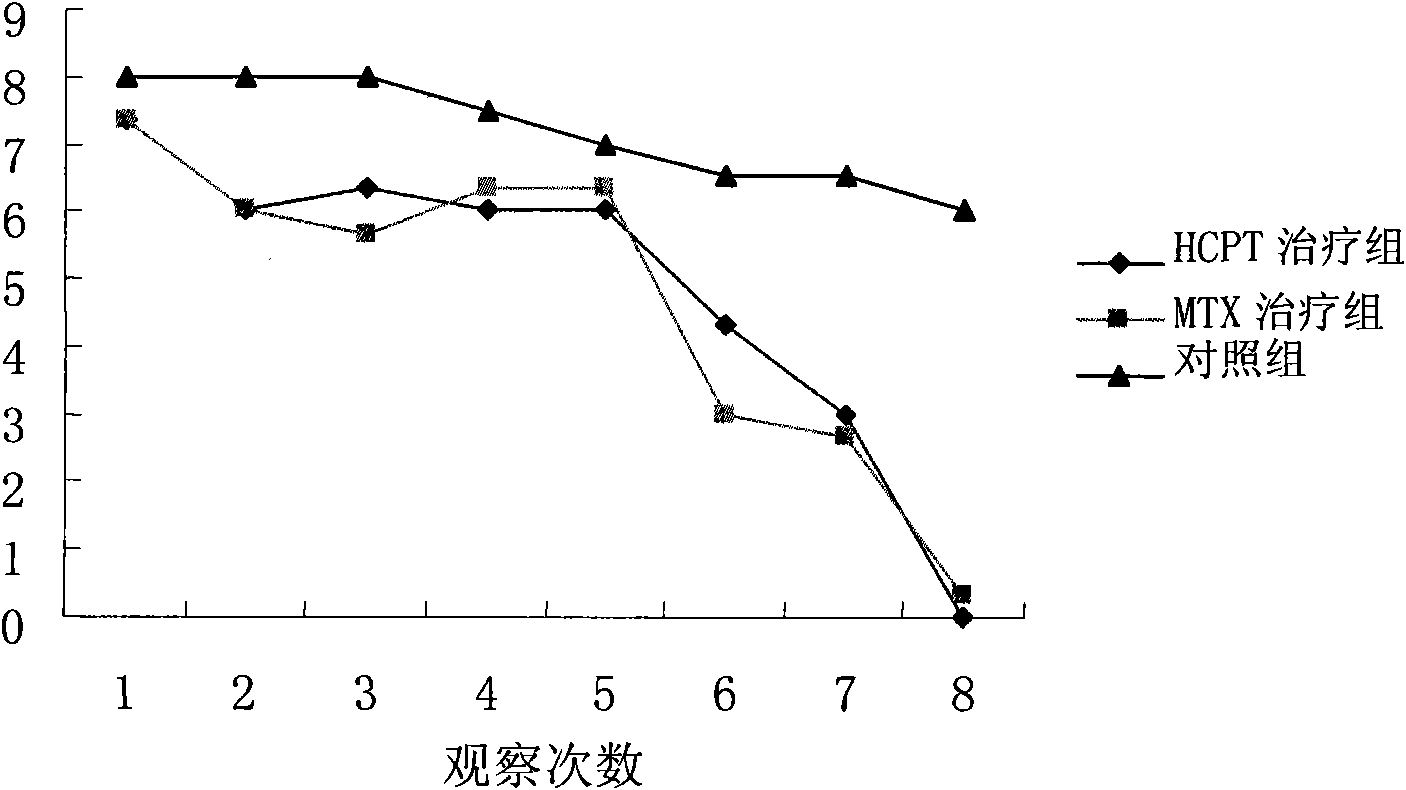
[0013] 6-week-old female Lewis rats were intradermally immunized with bC II at the base of the tail to establish a CIA model. A total of 36 affected mice were randomly divided into three groups. HCPT 2mg / kg was injected intraperitoneally every day, MTX positive control group 1mg / kg, 1 / week, intraperitoneal injection. At the same time, the normal saline control group was injected intraperitoneally with 200ul, 1 / day. The results of continuous treatment for 4 weeks showed that the body weight of the rats in the three groups showed a steady growth trend during the treatment period, with no significant difference, as shown in Figure 1. Compared with the blank control group, 2mg / kg hydroxycamptothecin treatment can significantly inhibit the joint inflammation of rats, reduce the degree of bone destruction, and significantly reduce the clinical and pathological scores of arthritis, as shown in Figure 2 and Table 1. This s...
Embodiment 2
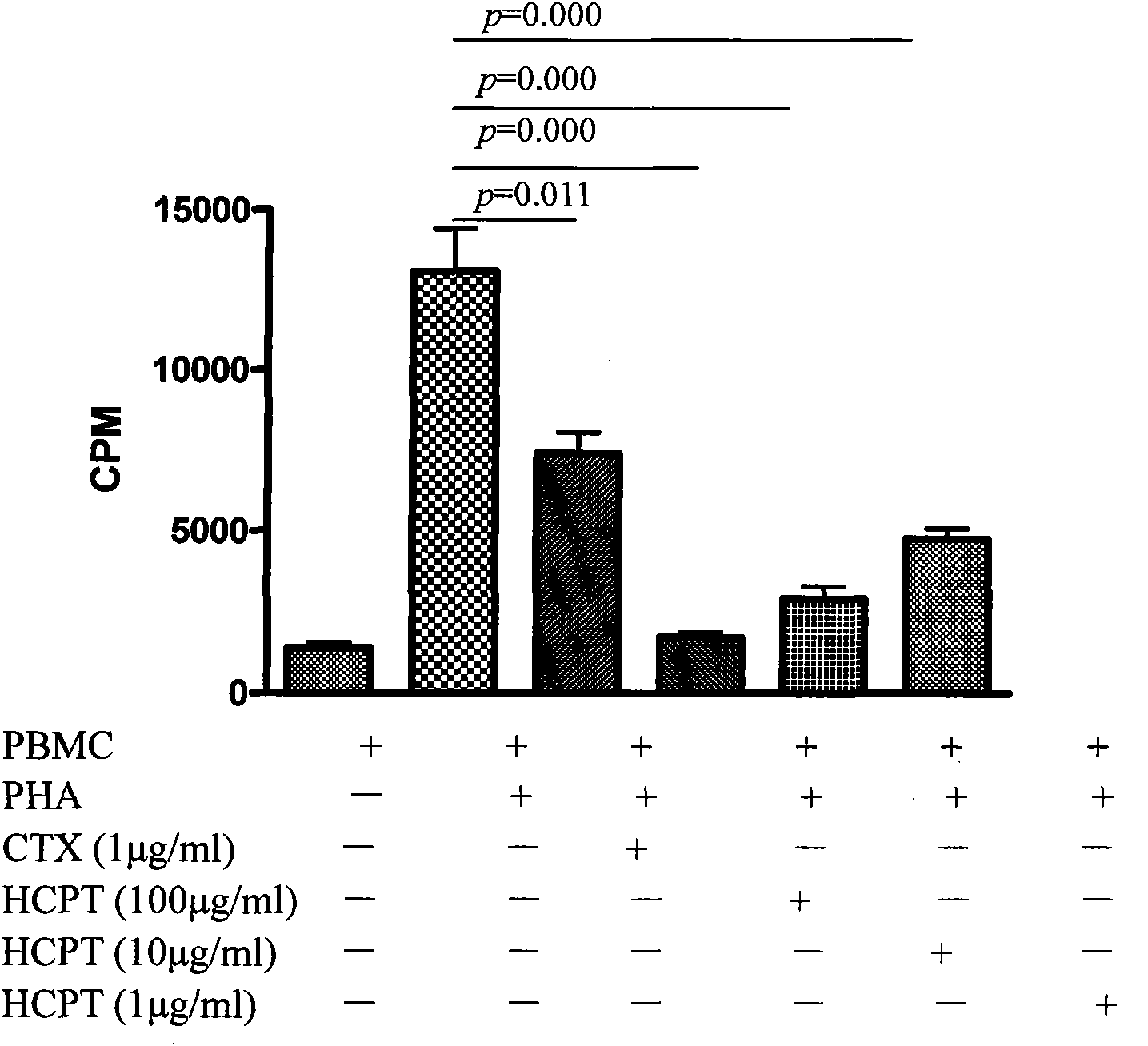
[0014] Example 2: Effects of Different Concentrations of Hydroxycamptothecin on PBMC Proliferation Inhibition
[0015] Isolation of PBMC from RA patients
[0016] ① Aseptically extract 15-20ml of anticoagulant blood from the patient's vein, and dilute it with PBS buffer. ②Equivalent volume of diluted blood was lightly spread in a 15ml sterile centrifuge tube containing human lymphocyte separation medium, centrifuged at 2000rpm for 20min. ③ Absorb the mononuclear cells in the middle layer, wash them twice with PBS (1500 rpm, centrifuge for 5 min), and discard the supernatant. ④ Mix well with RPMI-1640 culture medium containing 10% FBS.
[0017] The grouping of drugs on cell stimulation
[0018] Dosing in groups: ①Adjust the cell concentration, inoculate in a 96-well culture plate, and spread 2-4×10 cells in each well 5 / ml cell suspension, the total volume of each well is 200μl. Divide cells into negative control group, PHA control group (PHA working concentration is 2 μg / ...
Embodiment 3
[0020] Example 3: The effect of hydroxycamptothecin on PBMC-induced apoptosis and the effect on cell supernatant
[0021] Adjust the cell concentration, inoculate in a 24-well culture plate, and spread 2×10 per well 6 / ml cell suspension, the total volume of each well is 1.5ml. The cells were divided into negative control group, PHA control group, and HCPT stimulation group (working concentration: 1 μg / ml). After 72 hours of culture, the level of TGF-β1 in the cell supernatant was detected by enzyme-linked immunosorbent assay (ELISA), and Annexin V / PI flow cytometry to detect the effect of HCPT on cell apoptosis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com