Application of type II collagen allosteric peptide for treating rheumatoid arthritis with nasal mucosa medicine administration
A technology of allosteric peptide and nasal mucosa, which is applied in the application field of type II collagen allosteric peptide nasal mucosa administration in the treatment of rheumatoid arthritis, and can solve problems such as unacceptable, abnormal liver function, and difficult disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: Design and synthesis of polypeptide
[0024] The aforementioned research work (4-7) It has been proved that amino acids 267-270 in the CII polypeptide mainly bind to T cell receptors and activate T cells. In the experiment of the present invention, the CII263-272 prototype peptide and the CII allosteric peptide in which alanine was used to replace the corresponding amino acid were firstly synthesized by solid-phase method (Table 2). In order to improve the absorption rate, utilization and enhance the curative effect of the polypeptide, the amino terminal of each peptide is connected with the myristic acid gene during the synthesis, so as to facilitate the transport of the polypeptide into the cell. The decapeptides used in this study all contain more than 4 hydrophilic residues, such as lysine (K) and glutamic acid (E), which are easy to dissolve and absorb. These peptides do not contain easily degradable methionine (M), tryptophan (W), or easily deamina...
Embodiment 2
[0029] Example 2: The inhibitory effect of nasal mucosal administration of CII allosteric peptide of the present invention on experimental arthritis
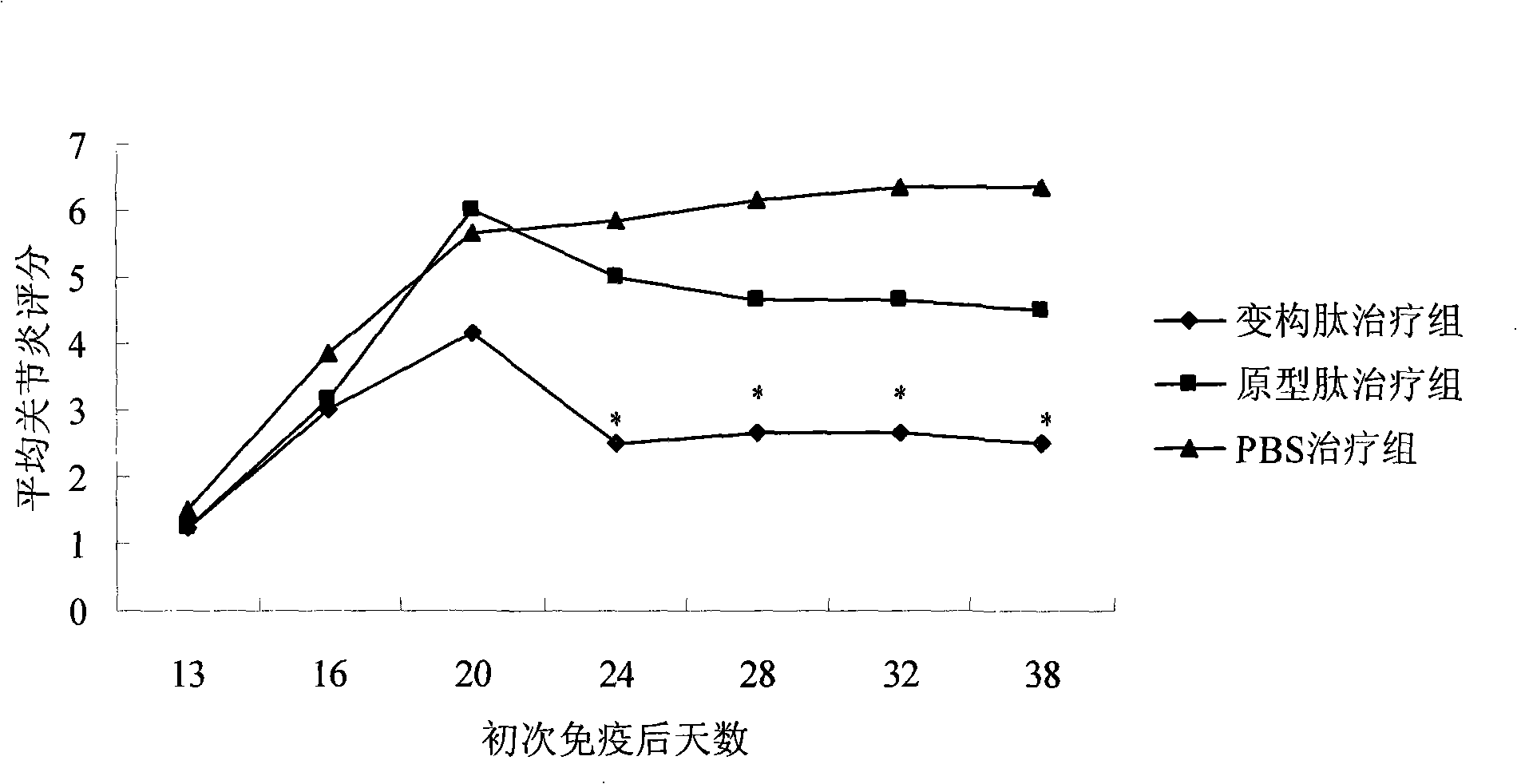
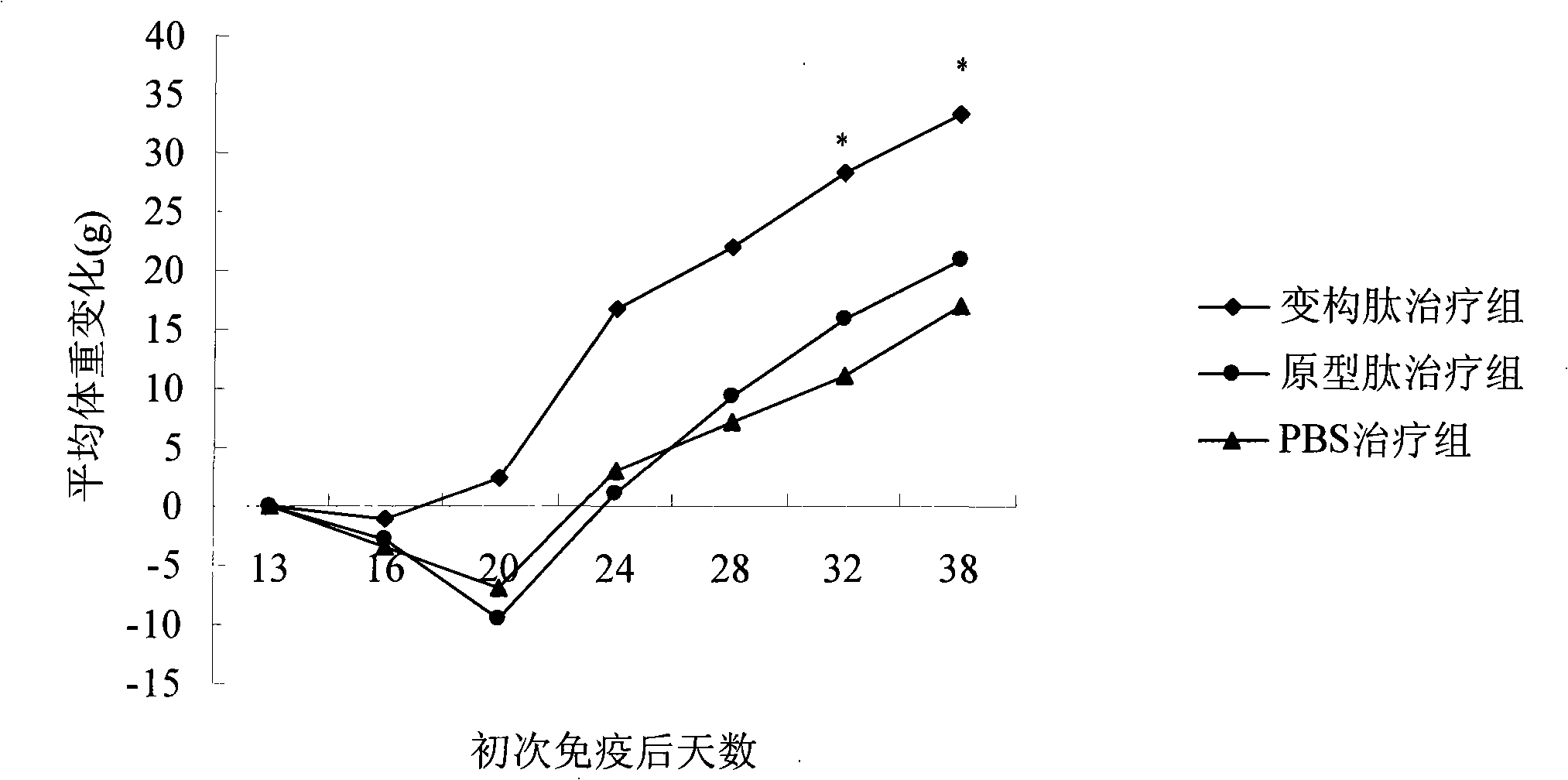
[0030] In the immune response leading to arthritis, antigen-presenting cells induce the activation of T cells through antigens, and promote the proliferation of T cells, causing increased levels of CII antibodies and cytokines (such as IFN-γ) in animals, which in turn leads to rheumatoid arthritis. The characteristic pathological changes of inflammation. Therefore, the inhibitory effect of non-T cell-binding peptides on arthritis can be understood by detecting the levels of cytokines in experimental animals. At the same time, the pathological section can also make a comprehensive judgment on the efficacy of the drug from the perspective of histology.
[0031]In the animal experiment scheme of the present invention, collagen-induced arthritis (CIA) in Lewis rats was induced with bovine CII, and then the arthritic rats were treat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com