Utilization method of linalool raw product refining raffinate synthesized by 6-methyl-5-heptenyl-2-one
A crude product, linalool technology, applied in the field of utilization of the refined raffinate of the crude product of linalool synthesized from 6-methyl-5-hepten-2-one, to reduce the amount of refined raffinate, improve the utilization value, The effect of reducing pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~8
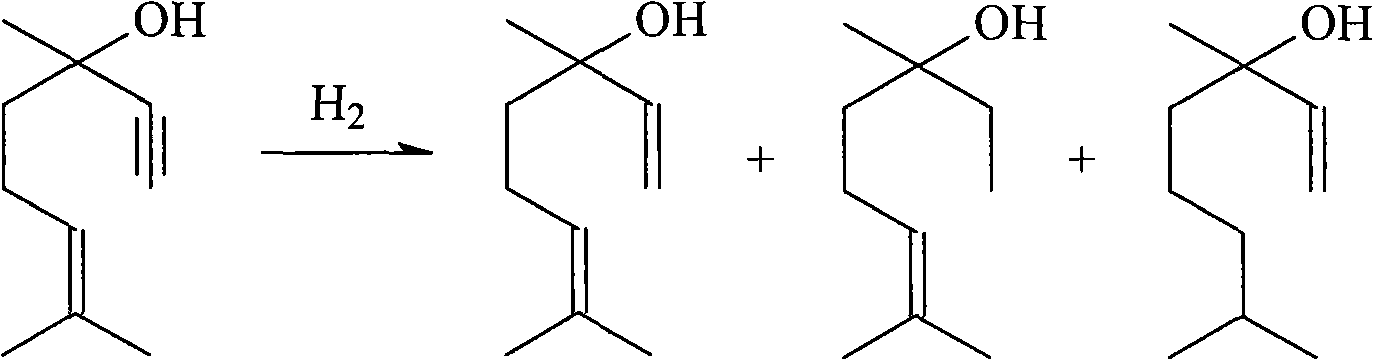
[0025] The test raw material is the refined raffinate of the crude product of linalool synthesized from isoprene by 6-methyl-5-hepten-2-one, which contains 24.9wt% of dihydrolinalool, 46.3 wt% of linalool wt%, dehydrolinalool 0.5wt%, (gas chromatographic analysis, area normalized quantification), others are mainly polymer impurities.
[0026] Place a rectification column in a 0.5L three-neck flask, and carry out rectification of the crude product refining raffinate to remove heavy component impurities. The inner diameter of the rectification column is 25mm, the length of the column is 700mm, and it is filled with φ3mm stainless steel packing. The vacuum of the system is obtained by a 2ZX-4 rotary vane vacuum pump. The feeding amount is 300mL, and the fraction at 80-90°C is collected at the top of the rectification column.
[0027] The rectification operating conditions of Examples 1 to 8 and the corresponding rectification yields are listed in Table 1, wherein the temperature...
Embodiment 9~18
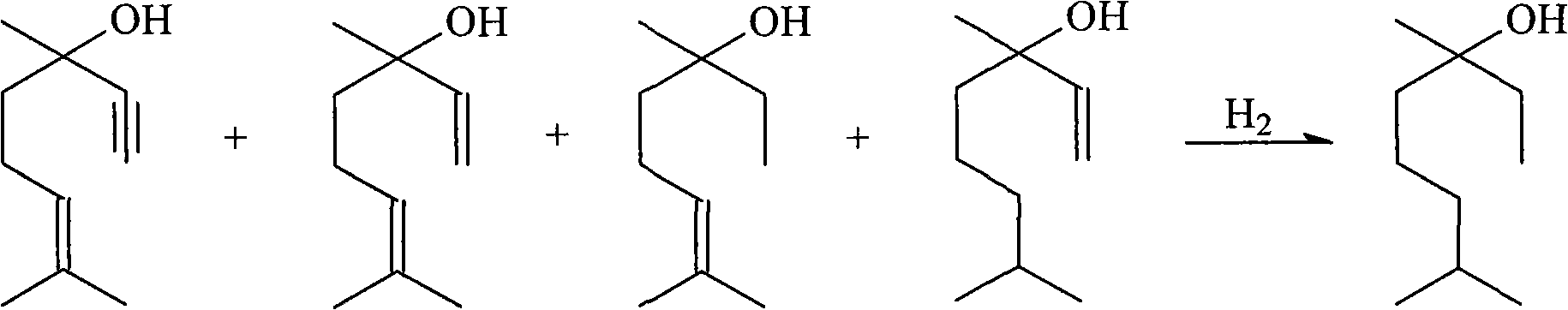
[0032] The 80~90 ℃ cut that embodiment 1~8 rectifying column top collects is mixed, as the raw material of hydrogenation reaction, wherein contains dihydrolinalool 38.4wt%, linalool 59.2wt%, dehydrolinalool 0.6 wt%.
[0033] The hydrogenation reaction is carried out in a 0.5L autoclave with stirring. The raw materials are put into the autoclave, and a catalyst is added. The catalyst is a supported catalyst with activated carbon as a carrier and palladium as an active component. The feeding amount of the raw material is 300mL, the system is replaced three times with 0.5MPa hydrogen, and then filled with hydrogen for hydrogenation reaction.
[0034] The specific hydrogenation process conditions and reaction yields of Examples 9 to 18 are shown in Table 2, wherein the amount of catalyst is based on the total amount of reactants, that is, the amount of hydrogenation reaction; the product content refers to the reaction liquid after the hydrogenation reaction is completed. The mass...
Embodiment 19~28
[0039] The hydrogenated products obtained in Examples 9-18 were cooled and mixed after separating the catalyst, wherein the mass fraction of the product tetrahydrolinalool was 94.0 wt%. The refining of tetrahydrolinalool was carried out by adopting batch rectification process, and refining was carried out in a 0.5L three-necked flask. The three-necked flask is equipped with a rectification column. The inner diameter of the rectification column is 25mm, the length of the column is 700mm, and it is filled with φ3mm stainless steel packing. The theoretical plate number of the rectification column is 16-17 with n-heptane-methylcyclohexane solution. The vacuum of the system is obtained by a 2ZX-4 rotary vane vacuum pump.
[0040] The feeding amount of each embodiment is 300mL.
[0041] First carry out vacuum rectification to remove light component impurities. The pressure at the top of the rectification column is controlled at 0.3-1.4KPa, and the light component impurities are the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com