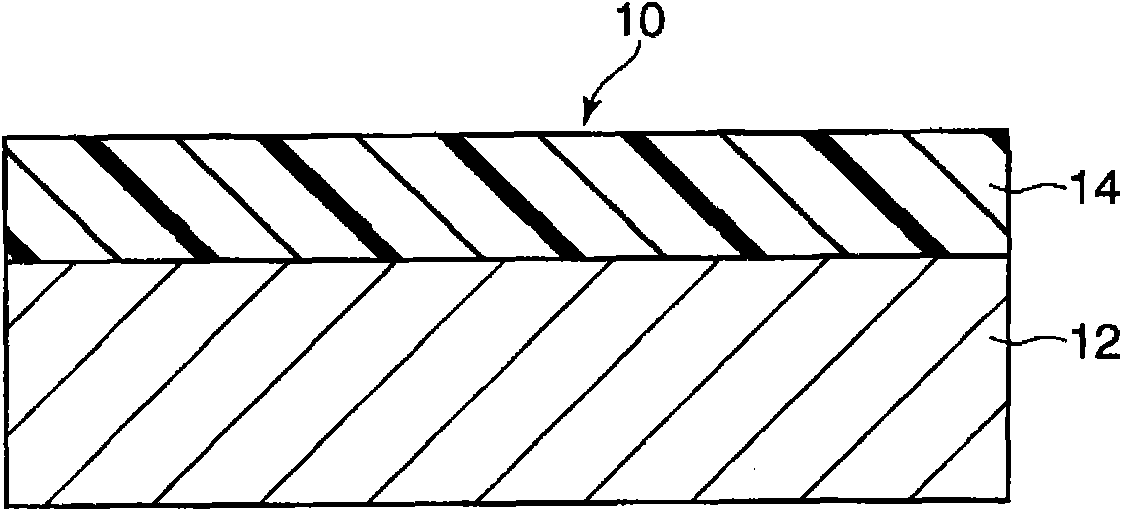
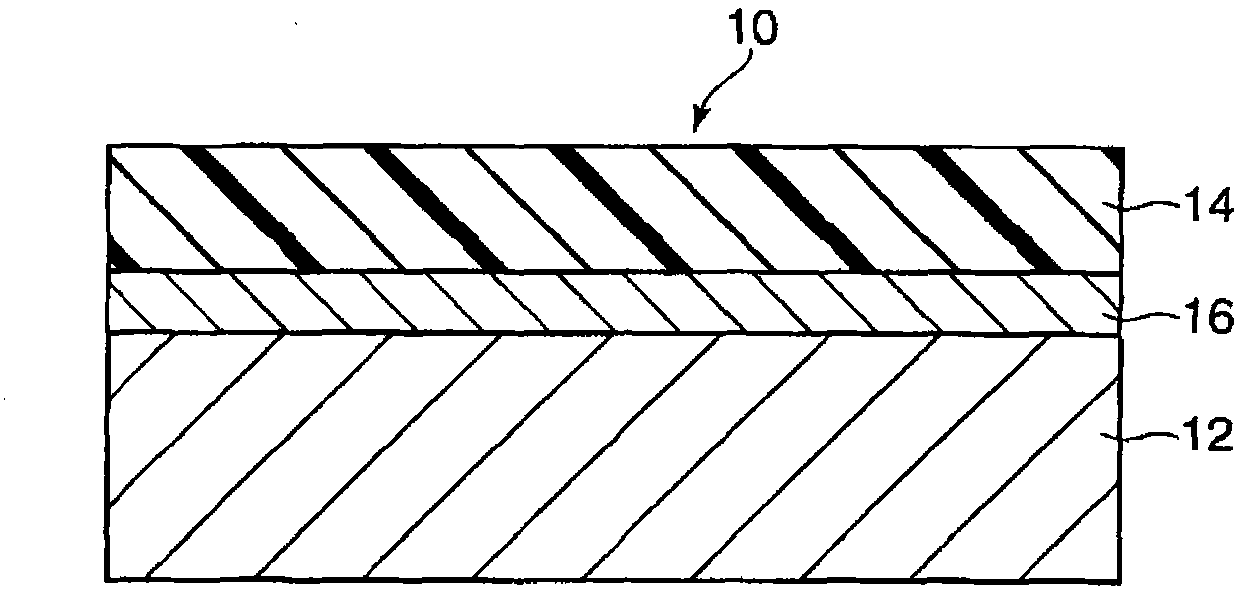
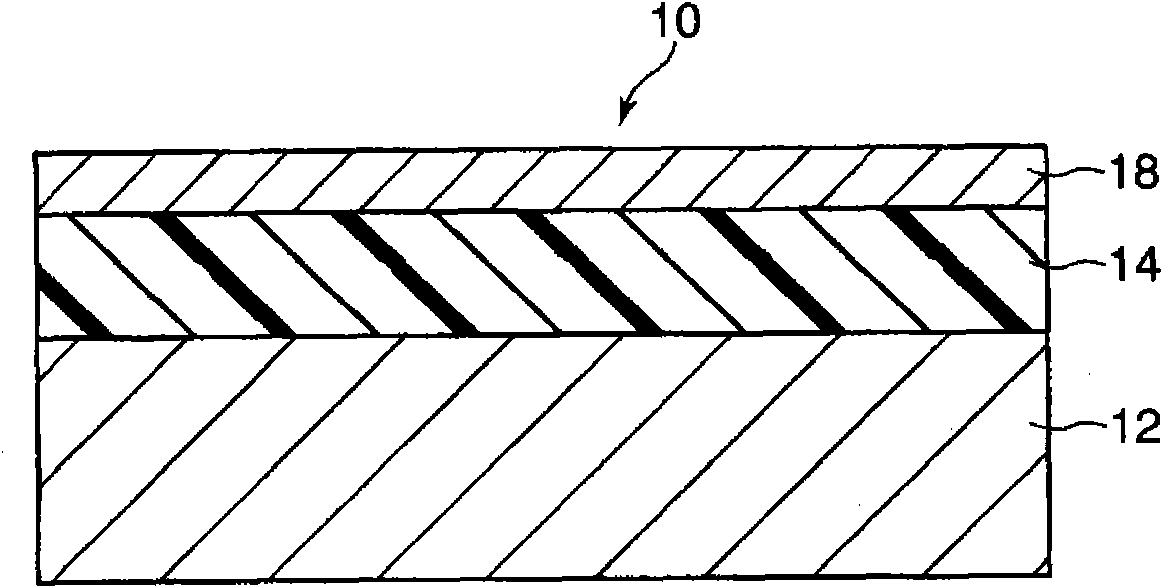
Electronic photographic sensitive body and manufacture method thereof
A technology of electrophotography and manufacturing method, applied in the directions of optics, electrography, instruments, etc., can solve the problems of insufficient sensitivity and insufficient compatibility, and achieve the effect of improving the sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0092] Hereinafter, the present invention will be described more specifically by way of examples. In addition, this invention is not limited to an Example.
[0093] [Synthesis of Pentabenzoquinone Derivatives]
Synthetic example 1
[0095] The synthesis of the pentabenzoquinone derivative represented by the chemical formula (1-1) proceeds as follows according to the following reaction formula (1-1).
[0096]
[0097] Reaction formula (1-1)
[0098] Under an inert gas, the THF (tetrahydrofuran) solution of 0.92 g (3.7 mmol) of the compound represented by formula (3-1) was cooled to -78 ° C, and a n-butyllithium hexane solution with a volume molar concentration of 1.6 M was added dropwise 2.33ml (3.7mmol), stirred for 1 hour. Then, 0.5 g (1.5 mmol) of the compound represented by the formula (2) was added, and the mixture was refluxed for 2 hours. Then, the THF solution was returned to room temperature, poured into water, extracted with ether, and the solvent was distilled off to obtain a residue.
[0099] Then, a solution of 9.2 g (40.5 mmol) of tin (II) chloride in 10 ml of dilute hydrochloric acid was added to the residue, followed by stirring at room temperature for 1 hour. Then, water was poured i...
Synthetic example 2
[0102] The synthesis of the pentabenzoquinone derivative represented by the chemical formula (1-2) proceeds as follows according to the following reaction formula (1-2).
[0103]
[0104] Reaction formula (1-2)
[0105]
[0106] Using 0.95 g of the compound (iodide) represented by the formula (3-2) in the same molar amount instead of the compound represented by the formula (3-1), the reaction was carried out in the same manner as in Synthesis Example 1, thereby obtaining the chemical formula 0.25 g of the pentabenzoquinone derivative shown in (1-2). The yield is 30%. Furthermore, the compound (iodide) represented by formula (3-2) is synthesize|combined from the aniline compound represented by formula (3-3) using a diazonium reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com