Nucleotide triphosphate with an electroactive label conjugated to the gamma phosphate
A technology of nucleoside triphosphate and nucleoside triphosphate conjugates, applied in the directions of labeling, combinatorial chemistry, organic chemistry, etc. used in chemical analysis, can solve the problems of time-consuming, increased cost, lengthy processing process, etc., and achieve simplified steps Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-F
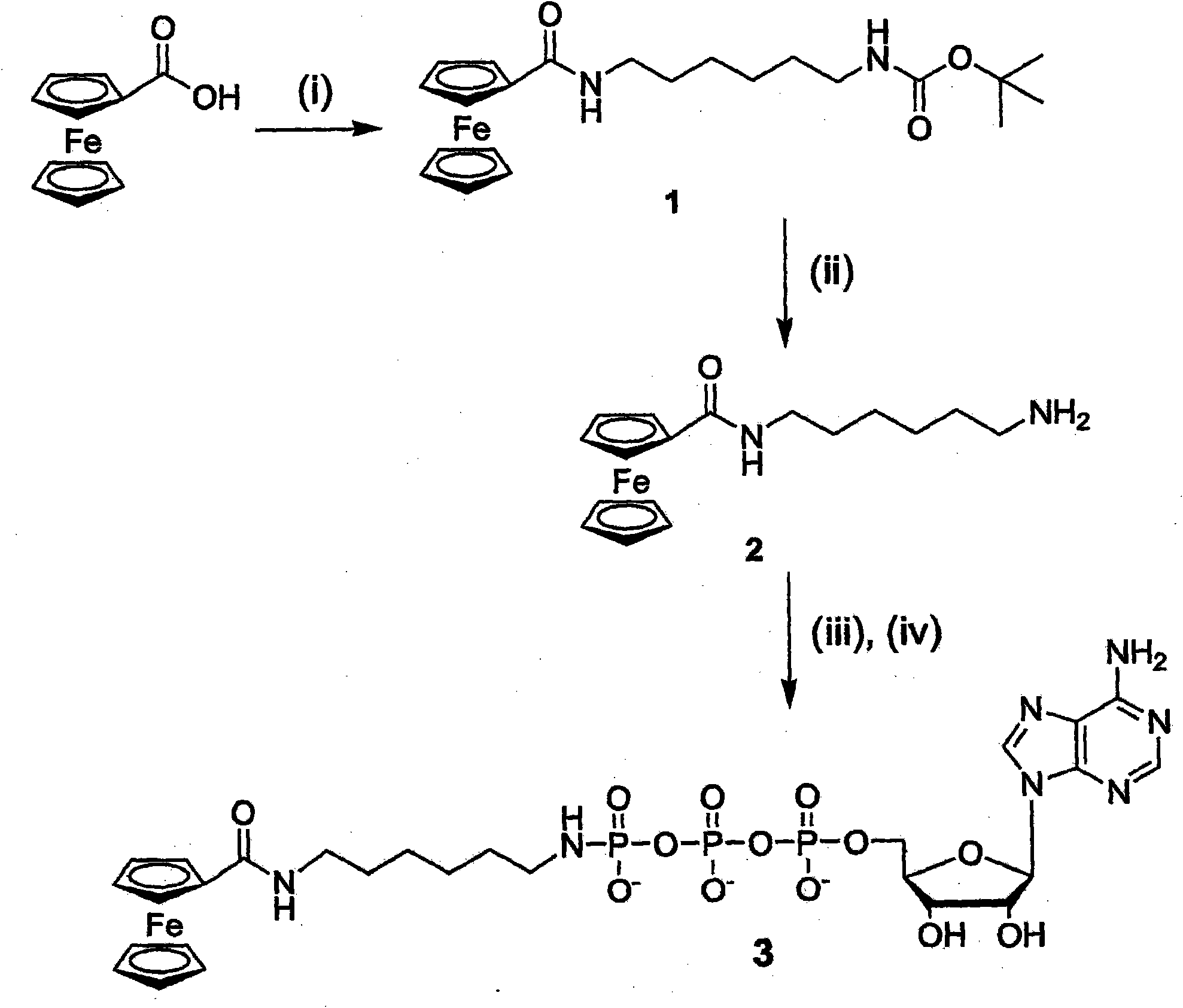
[0082] The synthesis of embodiment 1-Fc-ATP
[0083] Boc-NH(CH 2 ) 6 Preparation of N(H)COFc (Compound 1): Ferrocenecarboxylic acid (230 mg, 1 mmol) was dissolved in 20 mL of anhydrous DCM. Then, 1.2 equivalents of TEA (0.17 mL) and 1.2 equivalents of HBTU (455 mg) were added sequentially. After 30min, Boc-NH(CH 2 ) 6 NH 2 Add to the solution and continue stirring overnight. After the reaction was complete, the solvent was removed under vacuum, and the residue was purified by flash column chromatography on silica gel (DCM-MeOH, 95:5; R f = 0.25) to afford the desired compound as a yellow solid in 78% yield (334 mg). 1 H-NMR (δ, DMSO): 7.74 (t, 1H, J = 5.2Hz, NH-COFc), 6.78 (t, 1H, J = 5.4Hz, NH-Boc), 4.78 (s, 2H, Cp), 4.32(s, 2H, Cp), 4.14(s, 5H, Cp), 3.15(q, 2H, J=6.4Hz, CH 2 ), 2.90 (q, 2H, J=6.4Hz, CH 2 ), 1.23-1.52(m, 17H). 13 C( 1 H)-NMR (δ, DMSO): 168.57, 155.57, 77.27, 76.94, 69.73, 69.23, 68.06, 39.76, 38.54, 29.50, 29.47, 28.26, 26.17, 26.08. IR: v max...
Embodiment 2
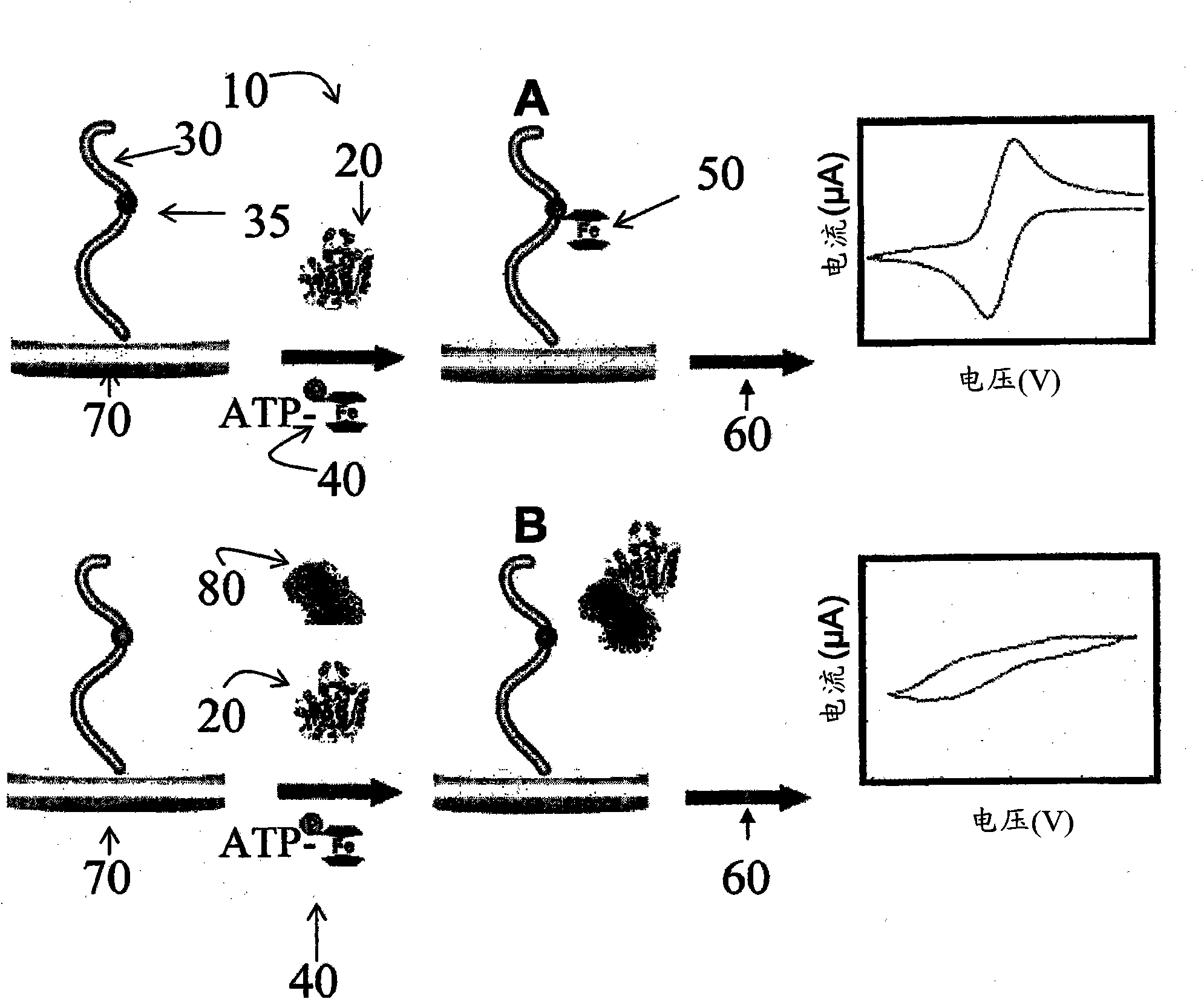
[0088] Example 2 - Electrochemical Detection of Protein Kinase C Phosphorylation
[0089] Cyclic voltammetry (CV) was performed using a CHInstruments 660 system (Austin, TX). DEP-chips with screen-printed gold electrodes (SPEs) were kindly donated by BioDevice Technology Ltd. (Ishikawa, Japan) and prepared as described in Li et al. Anal. Chem. 2005, 77, 5766-5769. The overall length of the SPE is 11mm, while the geometric area of the working electrode is 2.64mm 2 . The reference electrode is an Ag / AgCl paste electrode (Ag / AgCl past electrode), and the counter electrode is a carbon electrode.
[0090] 1 H, 13 C, 31 P NMR experiments were carried out on a Bruker Avance 500MHz spectrometer, and the chemical shifts were compared to residual DMSO (for 1 H is 2.50ppm and for 13 C is 39.52ppm) and H 2 O (4.79ppm). Mass spectrometry was performed using a Perkin Elmer-Sciex API365 instrument.
[0091] Reagents were purchased from Merek unless otherwise noted. All solutio...
Embodiment 3
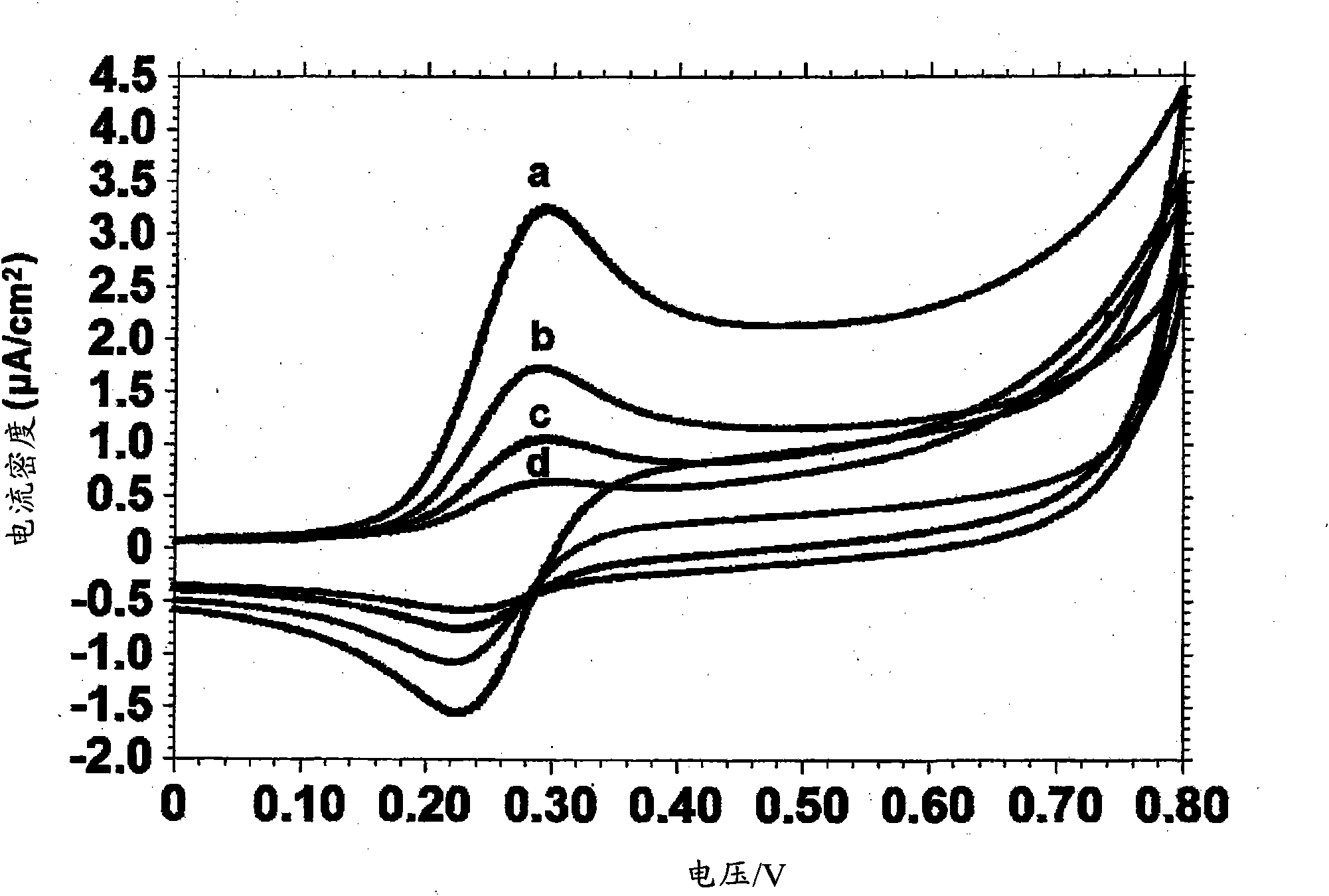
[0108] Example 3 - Casein Kinase 2 (CK2) and Tyrosine Kinase AbI1 and Detection of HER2 / ErbB2 phosphorylation
[0109] It has previously been demonstrated that the use of nucleoside triphosphate conjugates containing electroactively labeled γ-phosphate groups is suitable for detection of protein kinase C activity using electrochemical biosensing systems. In this example, the utility of nucleoside triphosphate conjugates was to detect another well-described protein serine / threonine kinase, casein kinase-2 (CK2), and two clinically important tyrosine kinases, AbI1 and HER2 / ErbB2 were used to evaluate this method for determining the potency of protein kinase inhibitors.
[0110] First for the kinase-specific peptide RRRDDDDSDDD of serine / threonine kinases 12 Enzyme modification of CK2 was evaluated by mass spectrometry using Fc-ATP as a co-substrate.
[0111] Figure 14 Shown for (A) CK2, (B) AbI1-T315I and (C) HER2 / ErbB2 using Applied Biosystems 4700 Proteomics Analyzer (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com