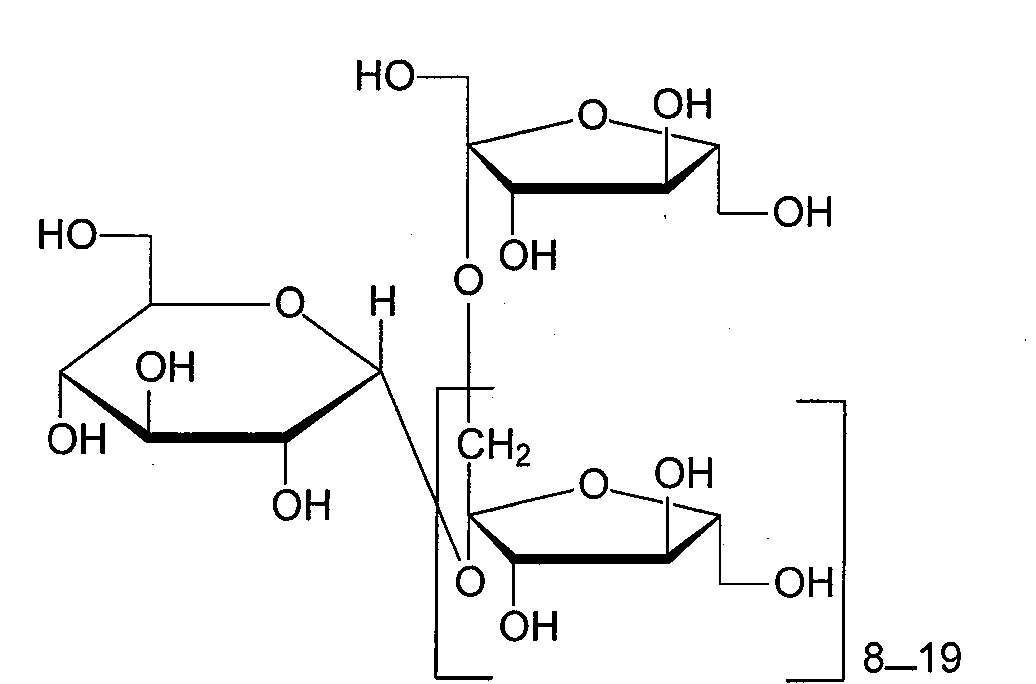
Radix Morindae officinalis extract, preparation method and applications thereof
A technology of extracts and drugs, which is applied in the field of new drugs, can solve the problems of antidepressant and anti-anxiety effects and applications of inulin-type 10-21 glycans that have not been seen, and inulin-types have not been found.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] For each monomer compound and extract of formula A, the dosage range for normal people is 0.1-40 mg / kg body weight / time, preferably 0.2-20 mg / kg body weight / time, based on the conversion of the body surface area of animals and humans. Example 1 Preparation of inulin-type 10-21 polysaccharides from Morinda officinalis
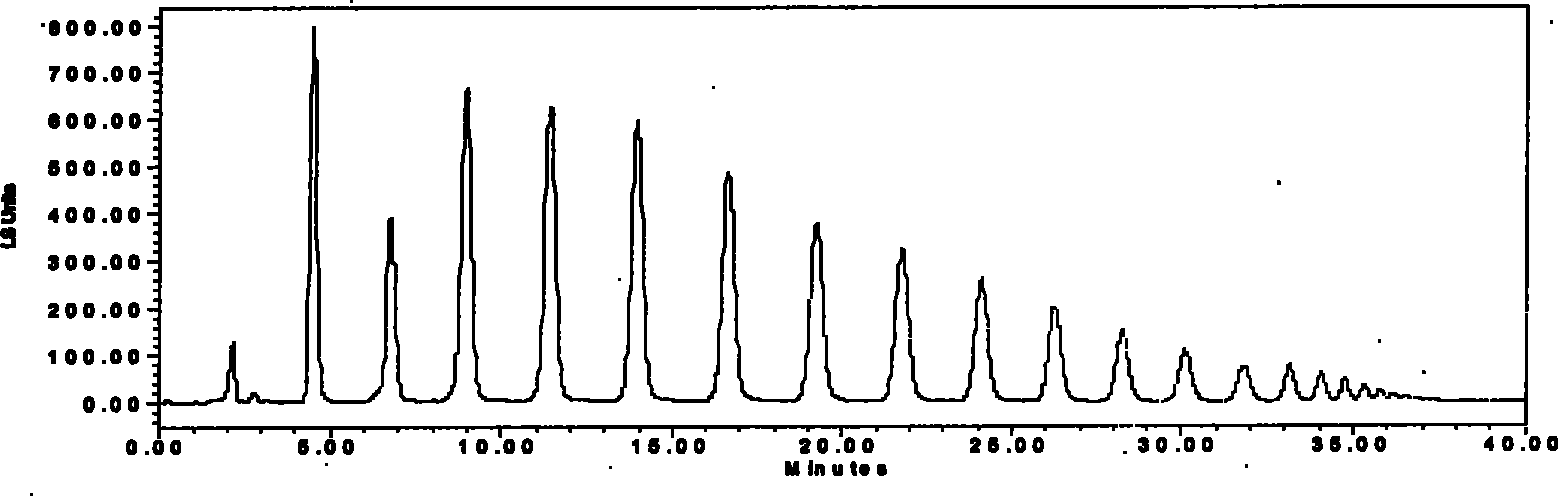
[0019] Take 500 grams of Morinda officinalis crude powder, heat and extract with 6 times the amount (W / W) of 30% ethanol, extract 4 times, each time for 1 hour, filter, combine the filtrates, concentrate to relative density D=1.05~1.10 ( 50 ℃) solution, put it at room temperature, filter, pass through a chromatographic column prepacked with activated carbon (the ratio of activated carbon to medicinal material is 1 / 1, W / W), first elute with 3% ethanol, and then use 50 % ethanol was eluted, and detected by high performance liquid phase (HPLC, system and conditions see Example 2), the fractions containing inulin-type 10-21 polysaccharides were collected, t...
Embodiment 2
[0024] Example 2 Preparation of inulin-type 10-21 polysaccharides from Jerusalem artichoke and Jerusalem artichoke
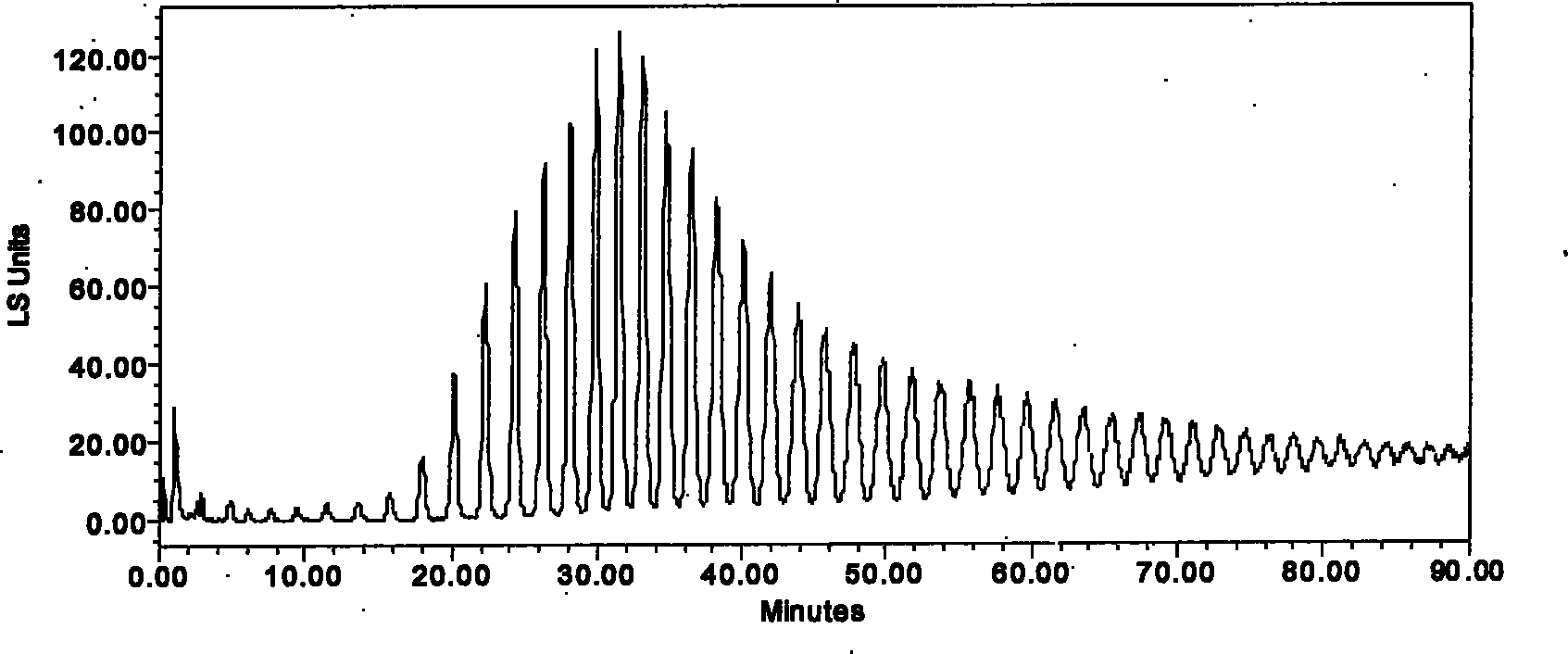
[0025] Get 500 grams of dried coarse powder of medicinal material Jerusalem artichoke, extract according to the extraction conditions of Example 1, obtain the Jerusalem artichoke extract containing formula A, and the yield is 10% to 30%; take another 500 grams of dried coarse powder of Jerusalem artichoke, and extract according to Carry out extraction under the extraction conditions of Example 1 to obtain the chrysanthemum yam extract containing formula A, with a yield of 8% to 20%;
[0026] According to the separation conditions of Example 1, the Jerusalem artichoke extract and the Jerusalem artichoke extract were separated and collected according to the peaks, and inulin-type 10-21 polysaccharides were also obtained. Figure three and picture Four.
[0027]
[0028]
Embodiment 3
[0029] Example 3 Structural identification of inulin-type 10-glycan
[0030] Inulin-type 10-glycan is a white solid, [α] 20D -19.5° (C=1.0, H 2 O). IRλmax cm -1 : 3425 (br s, OH), 2935, 2815, 1463 (w), 1085 (s), MALDI-TOF-MS m / z: 1638. 1 H-NMR (D 2 O)δ: 5.41 (1H d, J=3.7Hz; Glucose 1-H), 4.18~4.28 (multiple H, all are d peaks, J=8.2~8.3Hz, all are fructose 3-H), 3.85~4.15 (Multiple H, all are dd peaks, J=9.2~9.5Hz, all are fructose 4-H). 13 C-NMR (D 2 O) δ: 94.2 (glucose 1-C), 73.2 (glucose 2-C), 74.3 (glucose 3-C), 71.0 (glucose 4-C), 74.2 (glucose 5-C), 62.1 (glucose 6-C ). go through 1 H- 1 H COZY, 1 H- 13 C COZY, HMBC, HMQC comprehensive analysis, hydrolysis of inulin type 10 glycans, gas chromatography analysis (see Example 5 for the method), shows that the molar ratio of glucose to fructose is 1:9. Referring to the structural characteristics of such compounds (Cui Chengbin et al., "Inulin-type oligosaccharides 1 HNMR and 13 CNMR Research", Chinese Journal ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com