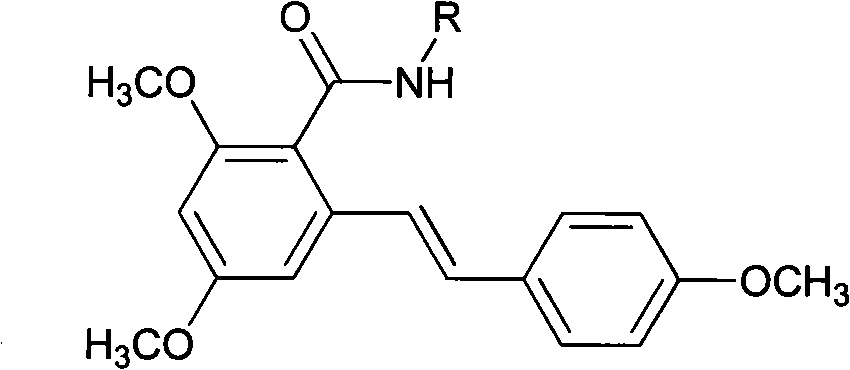
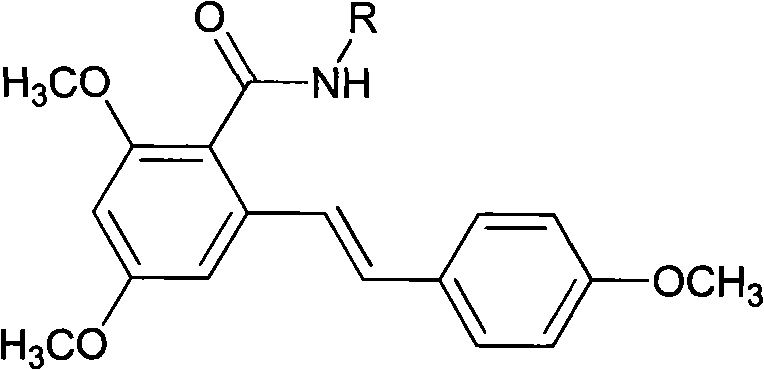
Resveratrol amide derivative and preparation method thereof
A technology of veratrol amide and retrol amide, which is applied in the field of medicine, can solve the problems of short metabolic cycle, side effects, and low anticancer activity, and achieve the effect of inhibiting growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Preparation of 3,5,4'-trimethoxy-2-carboxy resveratrol (compound 1)
[0024]
[0025] Add 20ml DMSO to a 50ml single-necked round bottom flask, add 3,5,4'-trimethoxy-2-formyl resveratrol (2.98g, 10mmol) under stirring, add NaH dropwise 2 PO 4 solution (0.5g NaH 2 PO 4 dissolved in 5ml water), stirred for 0.5h after the dropwise addition, and added NaClO dropwise 2 Solution (1.8g NaClO 2 dissolved in 20ml water), reacted at room temperature for 24h, and the reaction solution was poured into 5% NaHCO 3 In the solution, the aqueous phase was extracted with ethyl acetate, acidified with dilute hydrochloric acid, filtered, and the obtained solid was dissolved in dichloromethane, washed with saturated brine, dried, and recrystallized to obtain the target compound 3,5,4'-trimethoxy- 2-Carboxyresveratrol. Yield 72%, mp: 178-179°C. 1 H NMR (400MHz, CDCl 3 )δ (ppm): 3.78 (s, 6H), 3.85 (s, 3H), 6.54 (s, 1H), 6.91 (s, 1H), 6.92 (d, J=16.4, 1H), 6.97 (d, J =8....
Embodiment 2
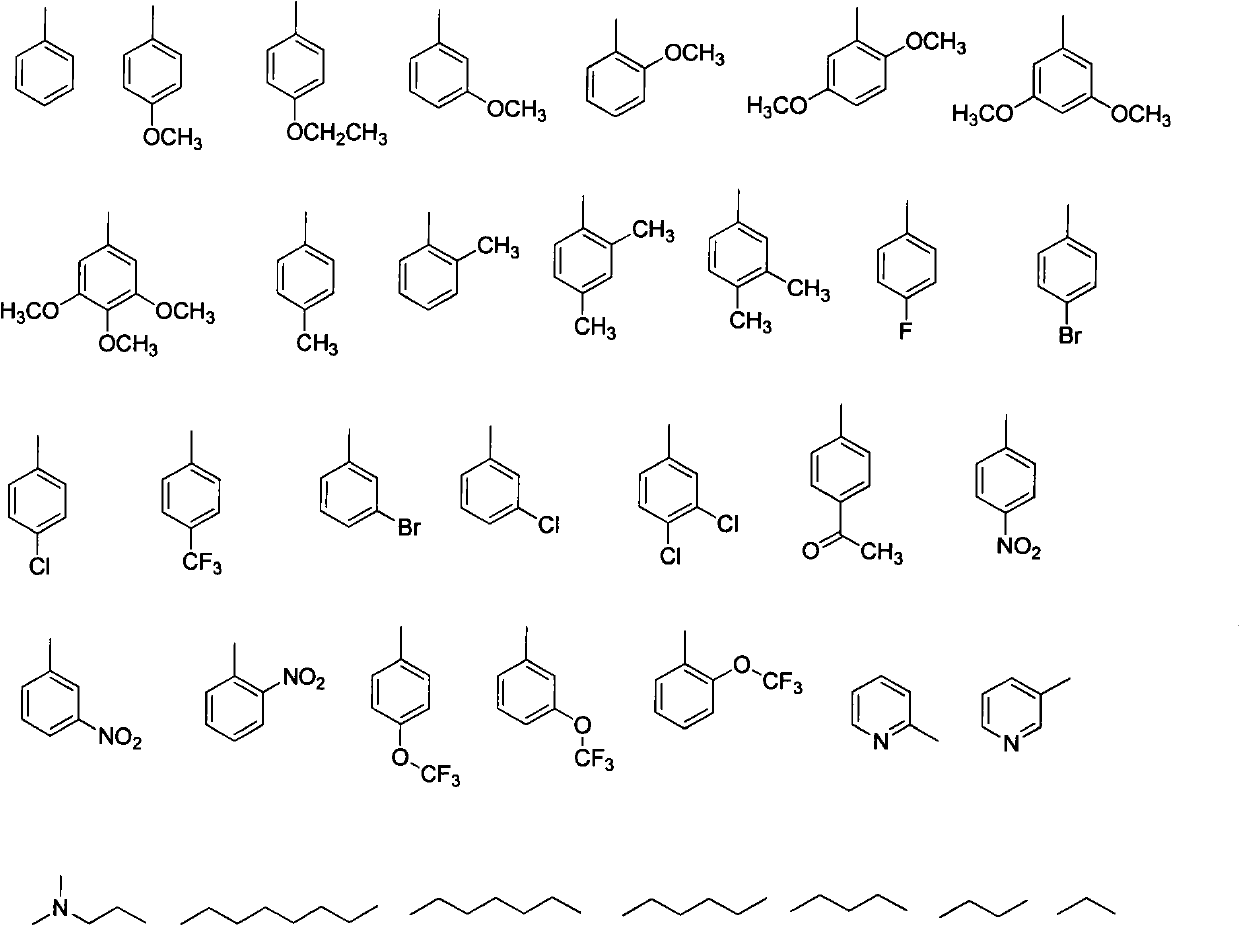
[0026] Example 2: Preparation of (trans)-2,4-dimethoxy-6-(-4-methoxystyryl)-N-phenylbenzamide (compound 2)
[0027]
[0028] Add 10ml of methylene chloride in a 50ml single-necked round bottom flask, add aniline (0.102g, 1.1mmol) and 3,5,4'-trimethoxy-2-carboxy resveratrol (0.314g, 1.0mmol) successively, EDC.HCl (0.287g, 1.5mmol), HOBt (0.068g, 0.5mmol), after reaction at room temperature for 12h, the solvent was evaporated under reduced pressure, the obtained paste solid was dissolved in ethyl acetate, washed with saturated brine and dried The target compound 1 was obtained by column chromatography. Yield 77%, mp: 119-120°C. 1 H NMR (400MHz, CDCl 3 )δ (ppm): 3.83 (s, 6H), 3.87 (s, 3H), 3.92 (s, 3H), 6.44 (s, 1H), 6.83 (s, 1H), 6.87 (d, J=8.8, 2H ), 6.92 (d, J=8.4, 2H), 7.05 (d, J=16.4, 1H), 7.26 (d, J=16.4, 1H), 7.43-7.48 (dd, 3H), 7.51 (s, 1H) , 7.57 (d, J=8.8, 2H). MS (ESI): 390.16 (C 24 h 23 NO 4 ,[M+H] + ).Anal.Calcd for C 24 h 23 NO 4 : C, 74.02; H, 5.95; N...
Embodiment 3
[0029] Example 3: Preparation of (trans)-2,4-dimethoxy-N-(4-methoxyphenyl)-6-(-4-methoxystyryl)benzamide (compound 3)
[0030]
[0031] The preparation method is the same as in Example 2. Substitute p-methoxyaniline for aniline to obtain (trans)-2,4-dimethoxy-N-(4-methoxyphenyl)-6-(-4-methoxystyryl)benzamide . Yield 79%, mp: 76-97°C. 1 H NMR (400MHz, CDCl 3 )δ (ppm): 3.83 (s, 6H), 3.87 (s, 3H), 3.92 (s, 3H), 6.44 (s, 1H), 6.83 (s, 1H), 6.87 (d, J=8.8, 2H ), 6.92 (d, J=8.4, 2H), 7.05 (d, J=16.4, 1H), 7.26 (d, J=16.4, 1H), 7.43 (d, J=8.8, 2H), 7.51 (s, 1H), 7.57 (d, J=8.8, 2H). MS (ESI): 420.17 (C 25 h 25 NO 5 ,[M+H] + ).Anal.Calcd for C 25 h 25 NO 5 : C, 71.58; H, 6.01; N, 3.34%; Found: C, 71.79; H, 6.00; N, 3.33%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com